Advances in the performance improvement strategies of tungsten oxide-based electrochromic smart windows
-
摘要: 電致變色智能窗能夠根據人們的喜好和天氣情況切換狀態調控陽光進入建筑物的透過率,并且不需要持續供能維持狀態,進而在保證建筑美觀的同時降低采光和制冷等方面的能源消耗。氧化鎢很早就被發現具有電致變色性能,其具有較大光學調制范圍和良好的穩定性,并經過近半個世紀的發展,氧化鎢基電致變色智能窗正逐步從實驗室走向實際應用。本文圍繞電致變色智能窗的性能評價指標,總結了提升氧化鎢電致變色性能的多種策略,包括制造氧空位、異類金屬元素摻雜、形貌和尺寸調控、電解質離子篩選和使用固態電解液,并且對各種策略進行了簡要的評價。最后,基于目前智能窗發展中存在的問題以及近期報道的具有前景的技術,對氧化鎢基電致變色智能窗的發展進行了展望。Abstract: The national strategic goal of “carbon peak and carbon neutrality” can be achieved without lowering living standards by the immediate development of energy saving devices. Where and how to use energy saving devices also must be considered. Energy consumption for building operations occupies a very large proportion of the total energy consumption, with over half of the building operation energy consumption being used for heating and cooling. Electrochromic smart windows can adjust the transmittance of solar radiation into a building by regulating them according to people’s preferences or weather conditions, thereby reducing energy usage. Because electrochromic smart windows use the dual injection of ions and electrons to cause polarization absorption of the material and optical modulation to block solar radiation, they do not require a continuous energy supply to maintain the state, thereby reducing the energy consumption for lighting and cooling while ensuring the building’s aesthetics. Electrochromic materials are the most important part of electrochromic smart windows. Tungsten oxide is a popular electrochromic material and is considered a promising material for electrochromic applications. Tungsten oxide has a large optical modulation range and good stability. After nearly half a century of development, tungsten oxide-based electrochromic smart windows are gradually moving from the laboratory to practical applications. This review will introduce some performance evaluation standards of electrochromic smart windows, including optical modulation range, response time, coloration efficiency, and stability. Based on the performance evaluation standard of electrochromic smart windows, this review provides a summary of several strategies to improve the electrochromic performance of tungsten oxide and presents evaluations of the strategies’ advantages and shortcomings, including the fabrication of oxygen vacancies, doping of heterogeneous metal elements, morphology and size regulation, electrolyte ion screening, and the use of solid electrolytes. Introducing oxygen vacancies in tungsten oxide can improve the optical modulation range; however, it may affect the stability of tungsten oxide. Doping of heterogeneous metal elements can enhance the coloration efficiency at the cost of prolonging the response time. Adjusting morphology and size can shorten the time of electrochromic response; however, it is difficult to control both the morphology and the size of materials. Replacing the electrolyte ion can improve all properties if a suitable ion can be found. Using a solid electrolyte will broaden the scope of tungsten oxide application at the cost of degraded electrochromic properties. Finally, based on the existing problems in the development of electrochromic smart windows and the recently reported promising technologies, this review presents a projection of the development of tungsten oxide-based electrochromic smart windows.
-
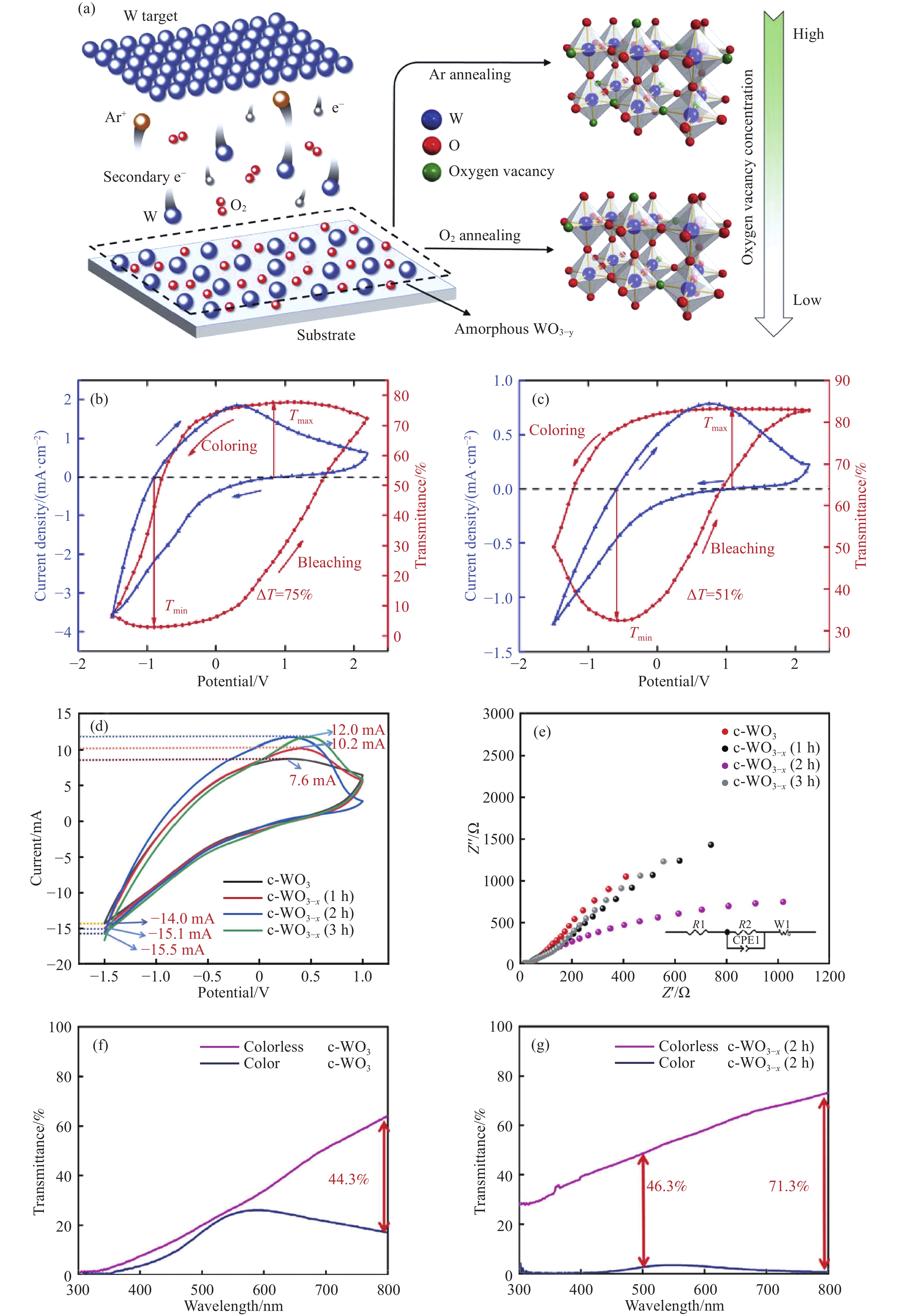
圖 1 (a) 磁控濺射法制備氧化鎢薄膜的示意圖及氬氣和氧氣氣氛退火所導致的晶體結構和氧空位變化[24];(b)~(c)分別是氧化鎢薄膜在氬氣和氧氣中退火后電壓在–1.5~2.2 V的循環伏安曲線(藍色線)和在650 nm處的原位光學透過率(紅色線). 藍色箭頭表示掃描方向, 紅色箭頭表示光學透過率變化趨勢[24];(d)~(e)分別為未浸泡處理(黑色線)、浸泡1(紅色線)、2(藍色線)和3 h后燒結(綠色線)的氧化鎢薄膜的循環伏安曲線圖和電化學阻抗譜[25];(f)未浸泡處理的氧化鎢著色態(藍色線)與褪色態(紫色線)的光學透過率[25];(g)浸泡處理2 h的氧化鎢著色態(藍色線)與褪色態(紫色線)的光學透過率[25]
Figure 1. (a) Illustration of the magnetron sputtered WO3-y thin film fabrication process and the crystal structure and oxygen vacancy change induced by Ar/O2 atmosphere annealing[24]; (b–c) cyclic voltammetry curves of tungsten oxide at –1.5–2.2 V after annealing in argon and oxygen (blue line) and in situ optical transmittance at 650 nm (red line), respectively. Blue arrow indicates the scanning direction and the red arrow indicates the optical transmittance switch[24]; (d–e) cyclic voltammetry curves electrochemical impedance spectroscopy data of the tungsten oxide unsoaked (black line), sintered after soaking for 1 (red line), 2 (blue line), and 3 h (green line)[25]; (f) optical transmittance of the unsoaked soaked tungsten oxide in the coloring state (blue line) and the bleaching state (purple line)[25]; (g) optical transmittance of the unsoaked soaked tungsten oxide sintered after soaking for 2 h in the coloring state (blue line) and the bleaching state (purple line)[25]
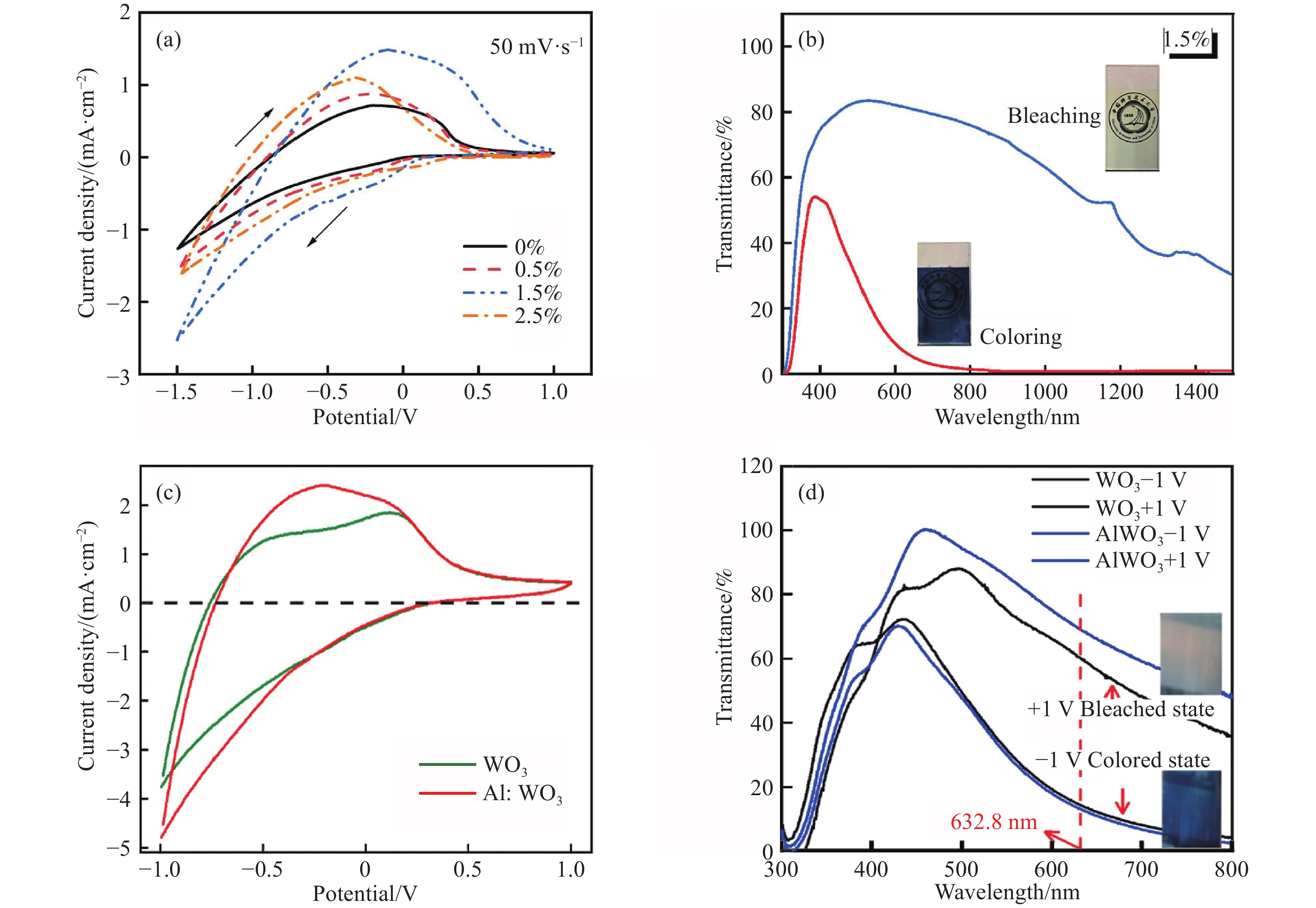
圖 2 (a) Co摻雜的氧化鎢薄膜在掃描速率為50 mV·s–1時的循環伏安曲線, 電解質為0.1 mol·L–1 LiClO4/PC [46];(b) 氧化鎢中Co原子摩爾摻雜濃度為1.5%時的著色態(紅色線)和漂白態(藍色線)的光學透過率, 插圖是對應狀態的照片[46];(c)未摻雜的氧化鎢(綠色線)與Al摻雜的氧化鎢(紅色線)在掃描速率為70 mV·s–1時的循環伏安曲線[47];(d)未摻雜的與Al摻雜的氧化鎢的著色態與褪色態的光學透過率[47]
Figure 2. (a) CV curves of Co-doped WO3 films at the scan rate of 50?mV·s–1 in 0.1?mol·L–1 LiClO4/PC electrolyte[46]; (b) optical transmittance of the coloring (red line) and bleaching (blue line) states at Co doping concentration of 1.5%. The inset are their corresponding graphs[46]; (c) cyclic voltammetry curves of undoped tungsten oxide (green line) and Al-doped (red line) tungsten oxide at a scanning rate of 70 mV·s–1[47]; (d) optical transmittance of the coloring and bleaching states of undoped and Al-doped tungsten oxide[47]
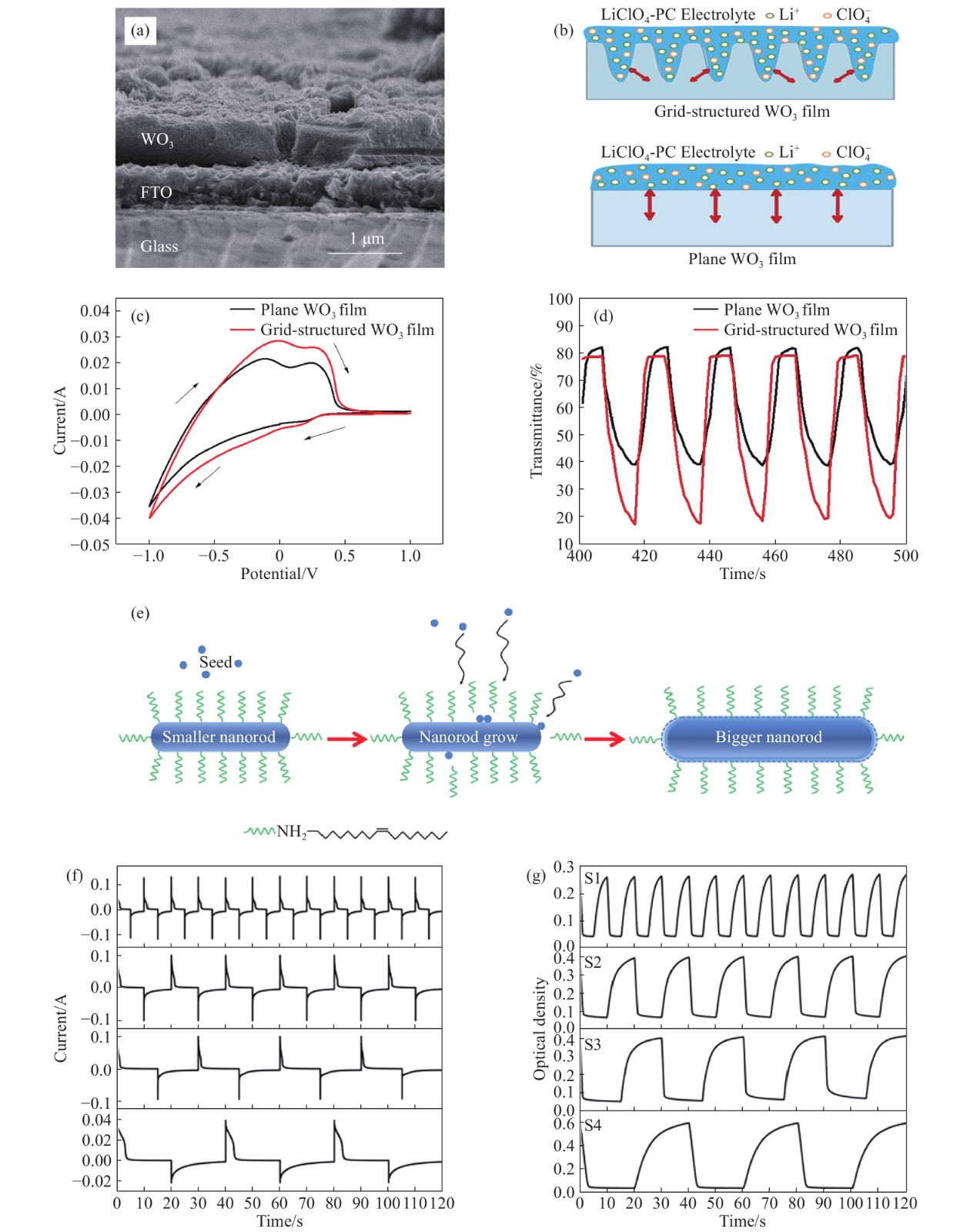
圖 3 (a)網狀凹槽氧化鎢薄膜的截面圖[48];(b)網狀凹槽結構與平面結構氧化鎢薄膜在LiClO4/PC電解液中的反應機理示意圖[48];(c)網狀凹槽結構(紅色線)與平面結構(黑色線)氧化鎢薄膜的循環伏安曲線[48];(d)網狀凹槽結構(紅色線)與平面結構(黑色線)氧化鎢薄膜時間與光學透過率的示意圖[48];(e)納米棒合成機理示意圖[49];(f)~(g)氧化鎢薄膜在狀態切換時的電流密度和在633 nm處對應的原位光學透過率隨時間變化的曲線. 納米棒按直徑從小到大排列, S1的納米棒尺寸最小, S4的尺寸最大[49]
Figure 3. (a) Sectional view of grid-structured WO3 films[48]; (b) mechanism diagram for EC reactions grid-structured of the grid-structured and plane WO3 films in LiClO4/PC electrolyte[48]; (c) cyclic voltammetry curves of the grid-structured (red line) and plane (black line) tungsten oxide films [48]; (d) time and optical transmittance of the grid-structured (red line) and plane (black line) tungsten oxide films[48]; (e) schematic diagram of the synthesis mechanisms of the nanorods[49]; (f–g) current density and corresponding in situ optical transmittance at 633 nm with time change of the tungsten oxide film during state transition. Nanorods are arranged in the order of size from small to large: S1 has the smallest size and S4 has the largest size[49]
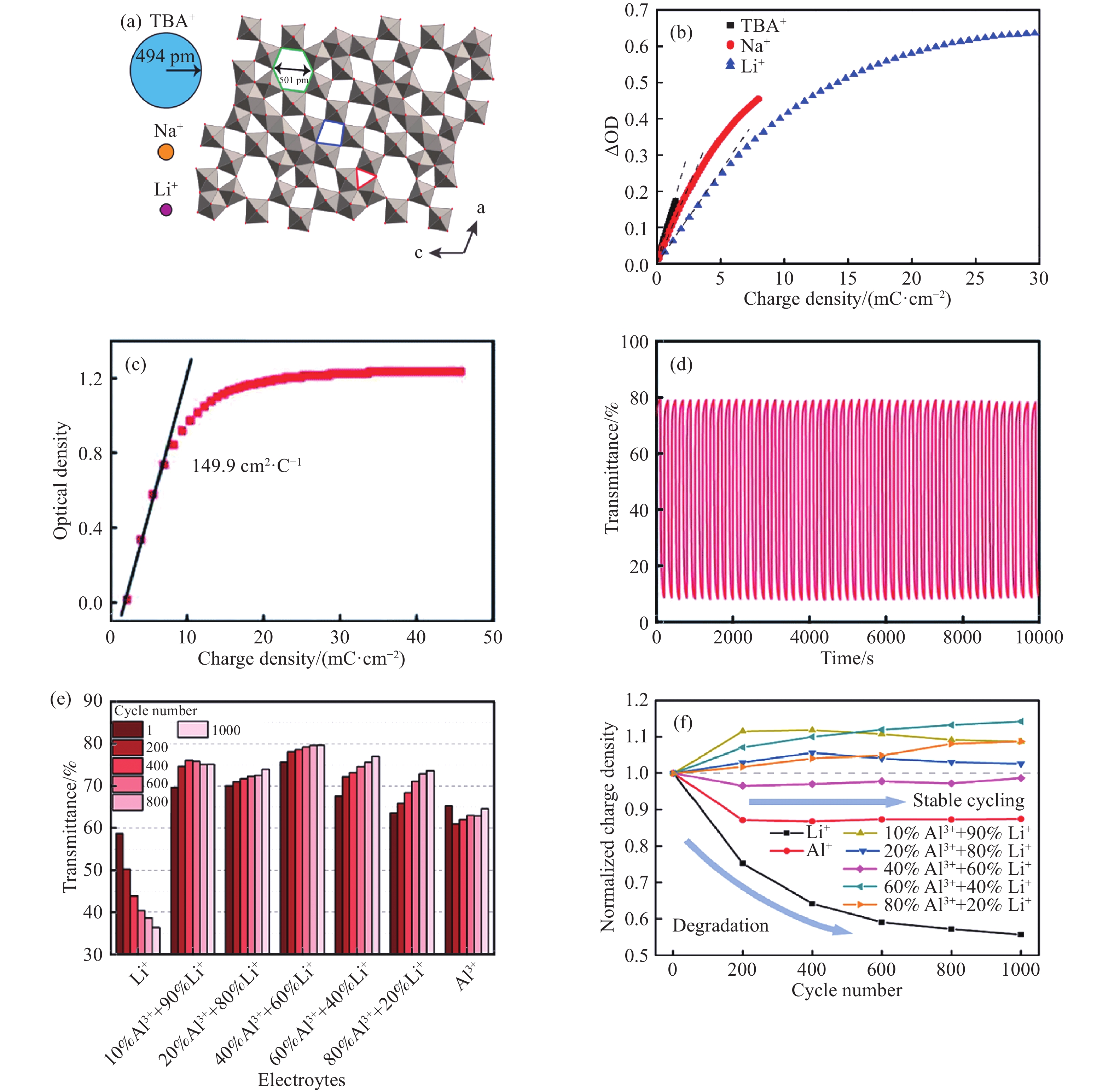
圖 4 (a) m-WO2.72晶體結構及TBA+、Na+和Li+的離子半徑的比例示意圖:(010)平面顯示了不同的間隙類型, 包括六角形空隙(綠線處)、四邊形空隙(藍線處)和三角形空隙(紅線處)[50];(b)氧化鋁涂層的氧化鎢薄膜在1200 nm處的光密度調制與累積恒流電荷密度的關系[50];(c)1 mol·L–1 AlCl3水溶液中800 nm處光密度隨電荷密度的變化[56];(d)交替使用電壓切換的氧化鎢原位透過率隨著色/漂白時間在660 nm處的變化[56];(e)在波長為550?nm處, 周期數為1、200、400、600、800和1000時, 施加±0.8?V電壓10?s?后的光學調制范圍[16];(f)在循環數為1、200、400、600、800和1000的計時電流循環中, 歸一化電荷密度隨循環數的變化規律[16]
Figure 4. (a) Scaled representations of the ionic radii of TBA+, Na+, and Li+ ions alongside the m-WO2.72 crystal structure: different interstitial sites are shown in the (010) plane, including hexagonal tunnels (green), trigonal cavities (red), and square windows (blue)[50]; (b) optical density modulation at 1200 nm plotted against accumulated galvanostatic charge density for 1 nm Al2O3-coated m-WO2.72 films with different electrolytes[50]; (c) variation of optical density vs charge density at 800 nm in 1 mol·L–1 AlCl3 aqueous electrolyte[56]; (d) In situ transmittance of tungsten oxide varies with the coloring/bleaching time at 660 nm using alternating voltage switches [56]; (e) transmittance modulation recorded in the cycle number of 1, 200, 400, 600, 800 and 1000 in the electrolytes with different proportion measured by CA with ±0.8?V for 10?s?at a wavelength of 550?nm[16]; (f) transmittance modulation of the electrolytes with different proportions measured at 1, 200, 400, 600, 800 and 1000 cycles by CA with ±0.8?V for 10?s?at a wavelength of 550?nm[16]; ?(g) evolution of normalized charge density versus cycle number corresponding to the CA cycles recorded at 1, 200, 400, 600, 800 and 1000[16]
表 1 典型的氧化鎢基電致變色智能窗性能
Table 1. Performance of typical tungsten oxide based electrochromic smart windows
Materials Morpholoy Electrolyte Optical contrast Response time (coloration/ bleaching)/s Coloration efficiency/(cm2·C–1) Stability Ref. WO2.77 — 1 mol·L–1 LiClO4/PC 75% 650 nm — 36.6 60% (after 1000 cycles) [24] WO2.91 — 1 mol·L–1 LiClO4/PC 51% 650 nm — 27.2 80% (after 1000 cycles) [24] c-WO3 Lamellar distribution 1 mol·L–1 LiClO4/PC 2.4%–44.3%
(500-800 nm)22.8/24 48.3 — [25] c-WO3–x (2 h) Lamellar distribution 1 mol·L–1 LiClO4/PC 46.3%–71.3%
(500–800 nm)11.6/20.7 102.8 — [25] WO2.9 Smooth and compact 1 mol·L–1 LiClO4/PC 35.2% 550 nm — 27.4 — [45] Li0.18W0.82O2.6 Smooth and compact 1 mol·L–1 LiClO4/PC 53.1% 550 nm — 41.6 — [45] WO3 Honeycomb-liked 0.1 mol·L–1 LiClO4/PC 30.8% 680 nm 9.8/2.4 18.7 Sharp degradation
(after 4000 cycles)[46] 1.5% Co-doped WO3 Nanowires array 0.1 mol·L–1 LiClO4/PC 75.4% 680 nm 27.6/11.8 65.7 No significant change
(after 4000 cycles)[46] WO3 Irregular aligned nanoplates 1 mol·L–1 LiClO4/PC 45.7% 632.8 nm 2.27/2.19 53.4 — [47] Al-doped WO3 Irregular aligned nanoplates 1 mol·L–1 LiClO4/PC 55.9% 632.8 nm 1.23/1.01 148.1 — [47] WO3 Grid-structured 1 mol·L–1 LiClO4/PC 64.3% 550 nm 1.27/0.93 71.8 — [48] WO3 plane 1 mol·L–1 LiClO4/PC 40.3% 550 nm 2.34/2.18 58.8 — [48] WO3 Nanorods
38 nm×3.2 nm0.5 mol·L–1 H2SO4 22% 633 nm 1.9/1.0 — No significant difference
(after 1000 cycles)[49] WO3 Nanorods
89 nm×4.5 nm0.5 mol·L–1 H2SO4 49% 633 nm 9.7/2.9 — No significant difference
(after 1000 cycles)[49] m-WO2.72 Nanorods 0.5 mol·L–1 LiClO4/PC — — 49 — [50] m-WO2.72 Nanorods 0.5 mol·L–1 NaClO4/PC — — 81 — [50] h-WO3 Nanorods 1 mol·L–1 AlCl3/aqueous 81.5% for the
maximum value9/14 149.9 — [56] a-WO3 Porous structure LiClO4/PC 79.6% 550 nm 14.4/6.8 20.4 Decreased significantly
(after 1000 cycles)[16] a-WO3 Porous structure AlClO4/PC 89.4% 550 nm 13.5/35.6 13.6 No obvious degradation
(after 1000 cycles)[16] a-WO3 Porous structure 10%AlClO4+
90%LiClO4/PC89.7% 550 nm 11.9/14.5 15.3 No obvious degradation
(after 1000 cycles)[16] tungsten oxide and Prussian blue Numerous cracks Solid-K+ 49.0% 633 nm 6.83/13.91 138.8 No degradation
(after 100 cycles)[60] tungsten oxide and Prussian blue Numerous cracks Gel-K+ 64.9% 633 nm 2.75/2.48 88.68 No degradation
(after 100 cycles)[60] WO3 — PEDOT:PSS-H+ more than 90% 650 nm 0.7/7.1 109 less than 10% Degradation
(after 3000 cycles)[61] www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] China Building Energy Efficiency Association. China building energy consumption annual report 2020. Build Energy Effic, 2021, 49(2): 1中國建筑節能協會. 中國建筑能耗研究報告2020. 建筑節能, 2021, 49(2):1 [2] Wang Y P, Na W, Tian Y P, et al. Characteristics of energy intensity of residential buildings including refrigeration, air conditioning and heating in China. Refrigeration, 2021, 40(4): 57 doi: 10.3969/J.ISSN.1005-9180.2021.04.011王云鵬, 那威, 田亞鵬, 等. 包含制冷、空調和采暖的我國住宅建筑能耗強度特征. 制冷, 2021, 40(4):57 doi: 10.3969/J.ISSN.1005-9180.2021.04.011 [3] Dun M, Wu L F. Forecasting the building energy consumption in China using grey model. Environ Process, 2020, 7(3): 1009 doi: 10.1007/s40710-020-00438-3 [4] Evangelisti L, Guattari C, Asdrubali F, et al. An experimental investigation of the thermal performance of a building solar shading device. J Build Eng, 2020, 28: 101089 doi: 10.1016/j.jobe.2019.101089 [5] Deb S K. A novel electrophotographic system. Appl Opt, 1969, 8(101): 192 [6] Buckner H B, Perry N H. In situ optical absorption studies of point defect kinetics and thermodynamics in oxide thin films. Adv Mater Interfaces, 2019, 6(15): 1900496 doi: 10.1002/admi.201900496 [7] Shimizu I, Shizukuishi M, Inoue E. Solid-state electrochromic device consisting of amorphous WO3 and Cr2O3. J Appl Phys, 1979, 50(6): 4027 doi: 10.1063/1.326483 [8] Lampert C M. Electrochromic materials and devices for energy efficient windows. Sol Energy Mater, 1984, 11(1-2): 1 doi: 10.1016/0165-1633(84)90024-8 [9] Deb S K. Optical and photoelectric properties and colour centres in thin films of tungsten oxide. Philos Mag A J Theor Exp Appl Phys, 1973, 27(4): 801 [10] Faughnan B W, Crandall R S, Heyman P M. Electrochromism in WO3 amorphous films. RCA Rev, 1975, 36(1): 177 [11] Svensson J, Granqvist C G. Electrochromic coatings for smart windows // Proceedings Volume 0502, Optical Materials Technology for Energy Efficiency and Solar Energy Conversion III. San Diego, 1984: 30 [12] Liu Y B, Wang J X, Xiao X D, et al. Synthesis of high-performance electrochromic thin films by a low-cost method. Ceram Int, 2021, 47(6): 7837 doi: 10.1016/j.ceramint.2020.11.130 [13] Wang Z, Gong W B, Wang X Y, et al. Remarkable near-infrared electrochromism in tungsten oxide driven by interlayer water-induced battery-to-pseudocapacitor transition. ACS Appl Mater Interfaces, 2020, 12(30): 33917 doi: 10.1021/acsami.0c08270 [14] Kim K W, Yun T Y, You S H, et al. Extremely fast electrochromic supercapacitors based on mesoporous WO3 prepared by an evaporation-induced self-assembly. NPG Asia Mater, 2020, 12: 84 doi: 10.1038/s41427-020-00257-w [15] Sun T H, Liu H J, Fu G Y, et al. Measuring and improving response time of WO3 thin film electrochromic devices. Chin J Liq Cryst Disp, 2021, 36(5): 641 doi: 10.37188/CJLCD.2020-0293孫天皓, 劉紅均, 伏桂月, 等. WO3薄膜電致變色器件的響應時間測試及其改善方案. 液晶與顯示, 2021, 36(5):641 doi: 10.37188/CJLCD.2020-0293 [16] Yu H, Guo J J, Wang C, et al. High performance in electrochromic amorphous WOx film with long-term stability and tunable switching times via Al/Li-ions intercalation/deintercalation. Electrochimica Acta, 2019, 318: 644 doi: 10.1016/j.electacta.2019.06.129 [17] Wang S, Xu H B, Hao T T, et al. In situ XRD and operando spectra-electrochemical investigation of tetragonal WO3-x nanowire networks for electrochromic supercapacitors. NPG Asia Mater, 2021, 13: 51 doi: 10.1038/s41427-021-00319-7 [18] Jo M H, Kim K H, Ahn H J. P-doped carbon quantum dot graft-functionalized amorphous WO3 for stable and flexible electrochromic energy-storage devices. Chem Eng J, 2022, 445: 136826 doi: 10.1016/j.cej.2022.136826 [19] Zhang S L, Cao S, Zhang T R, et al. Monoclinic oxygen-deficient tungsten oxide nanowires for dynamic and independent control of near-infrared and visible light transmittance. Mater Horiz, 2018, 5(2): 291 doi: 10.1039/C7MH01128H [20] Park S, Park H S, Dao T T, et al. Solvothermal synthesis of oxygen deficient tungsten oxide nano-particle for dual band electrochromic devices. Sol Energy Mater Sol Cells, 2022, 242: 111759 doi: 10.1016/j.solmat.2022.111759 [21] Zhang S L, Cao S, Zhang T R, et al. Overcoming the technical challenges in Al anode-based electrochromic energy storage windows. Small Methods, 2020, 4(1): 1900545 doi: 10.1002/smtd.201900545 [22] Nguyen T H Q, Eberheim F, G?bel S, et al. Enhancing the spectroelectrochemical performance of WO3 films by use of structure-directing agents during film growth. Appl Sci, 2022, 12(5): 2327 doi: 10.3390/app12052327 [23] Zhong X L, Liu X Q, Diao X G. Electrochromic devices based on tungsten oxide and nickel oxide: A review. J Inorg Mater, 2021, 36(2): 128 doi: 10.15541/jim20200488鐘曉嵐, 劉雪晴, 刁訓剛. 基于氧化鎢和氧化鎳的電致變色器件研究進展. 無機材料學報, 2021, 36(2):128 doi: 10.15541/jim20200488 [24] Yu H, Guo J J, Wang C, et al. Essential role of oxygen vacancy in electrochromic performance and stability for WO3-y films induced by atmosphere annealing. Electrochimica Acta, 2020, 332: 135504 doi: 10.1016/j.electacta.2019.135504 [25] Li Z X, Liu Z F, Li J W, et al. The electrochromic properties of the film enhanced by introducing oxygen vacancies to crystalline tungsten oxide. Colloids Surf A Physicochem Eng Aspects, 2022, 641: 128615 doi: 10.1016/j.colsurfa.2022.128615 [26] Hasani A, Le Q V, Nguyen T P, et al. A thorough study on electrochromic properties of metal doped tungsten trioxide film prepared by a facile solution process. Electrochimica Acta, 2018, 283: 1195 doi: 10.1016/j.electacta.2018.07.050 [27] Zhou J L, Wei Y X, Luo G, et al. Electrochromic properties of vertically aligned Ni-doped WO3 nanostructure films and their application in complementary electrochromic devices. J Mater Chem C, 2016, 4(8): 1613 doi: 10.1039/C5TC03750F [28] Xie S J, Bi Z J, Chen Y B, et al. Electrodeposited Mo-doped WO3 film with large optical modulation and high areal capacitance toward electrochromic energy-storage applications. Appl Surf Sci, 2018, 459: 774 doi: 10.1016/j.apsusc.2018.08.045 [29] Pooyodying P, Ok J W, Son Y H, et al. Electrical and optical properties of electrochromic device with WO3: Mo film prepared by RF magnetron Co-sputtering. Opt Mater, 2021, 112: 110766 doi: 10.1016/j.optmat.2020.110766 [30] Li W L, Zhang J, Zheng Y H, et al. High performance electrochromic energy storage devices based on Mo-doped crystalline/amorphous WO3 core-shell structures. Sol Energy Mater Sol Cells, 2022, 235: 111488 doi: 10.1016/j.solmat.2021.111488 [31] Zhan Y, Tan M R J, Cheng X, et al. Ti-doped WO3 synthesized by a facile wet bath method for improved electrochromism. J Mater Chem C, 2017, 5(38): 9995 doi: 10.1039/C7TC02456H [32] Song Y, Zhang Z Y, Yan L M, et al. Electrodeposition of Ti-doped hierarchically mesoporous silica microspheres/tungsten oxide nanocrystallines hybrid films and their electrochromic performance. Nanomaterials, 2019, 9(12): 1795 doi: 10.3390/nano9121795 [33] Park H S, Park S, Song S H, et al. Effects of Ti-doping amount and annealing temperature on electrochromic performance of sol-gel derived WO3. RSC Adv, 2022, 12(27): 17401 doi: 10.1039/D2RA02247H [34] Park S, Thuy D T, Sarwar S, et al. Synergistic effects of Ti-doping induced porous networks on electrochromic performance of WO3 films. J Mater Chem C, 2020, 8(48): 17245 doi: 10.1039/D0TC04420B [35] Wang W Q, Yao Z J, Wang X L, et al. Niobium doped tungsten oxide mesoporous film with enhanced electrochromic and electrochemical energy storage properties. J Colloid Interface Sci, 2019, 535: 300 doi: 10.1016/j.jcis.2018.10.006 [36] Wang L S, Li D, Zhou Y L, et al. Optimization of hydrogen-ion storage performance of tungsten trioxide nanowires by niobium doping. Nanotechnology, 2022, 33(10): 105403 doi: 10.1088/1361-6528/ac3e8e [37] Olkun A, Pat S, Akkurt N, et al. Detailed transmittance analysis of high-performance SnO2-doped WO3 thin films in UV-Vis region for electrochromic devices. J Mater Sci:Mater Electron, 2020, 31(21): 19074 doi: 10.1007/s10854-020-04444-x [38] Luo G, Shen L Y, Zheng J M, et al. A europium ion doped WO3 film with the bi-functionality of enhanced electrochromic switching and tunable red emission. J Mater Chem C, 2017, 5(14): 3488 doi: 10.1039/C7TC00248C [39] Shen L Y, Luo G, Zheng J M, et al. Effect of pH on the electrochromic and photoluminescent properties of Eu doped WO3 film. Electrochimica Acta, 2018, 278: 263 doi: 10.1016/j.electacta.2018.05.033 [40] Kunyapat T, Xu F, Neate N, et al. Ce-doped bundled ultrafine diameter tungsten oxide nanowires with enhanced electrochromic performance. Nanoscale, 2018, 10(10): 4718 doi: 10.1039/C7NR08385H [41] Shen L Y, Zheng J M, Xu C Y. Enhanced electrochromic switches and tunable green fluorescence based on terbium ion doped WO3 films. Nanoscale, 2019, 11(47): 23049 doi: 10.1039/C9NR06125H [42] Bathe S R, Patil P S. WO3 thin films doped with Ru by facile chemical method with enhanced electrochromic properties for electrochromic window application. Mater Sci Eng B, 2020, 257: 114542 doi: 10.1016/j.mseb.2020.114542 [43] Yin Y, Lan C Y, Hu S M, et al. Effect of Gd-doping on electrochromic properties of sputter deposited WO3 films. J Alloys Compd, 2018, 739: 623 doi: 10.1016/j.jallcom.2017.12.290 [44] Zeb S, Sun G X, Nie Y, et al. Advanced developments in nonstoichiometric tungsten oxides for electrochromic applications. Mater Adv, 2021, 2(21): 6839 doi: 10.1039/D1MA00418B [45] Chang J Y, Chen Y C, Wang C M, et al. Electrochromic properties of lithium-doped tungsten oxide prepared by electron beam evaporation. Coatings, 2019, 9(3): 191 doi: 10.3390/coatings9030191 [46] Shen K, Sheng K, Wang Z T, et al. Cobalt ions doped tungsten oxide nanowires achieved vertically aligned nanostructure with enhanced electrochromic properties. Appl Surf Sci, 2020, 501: 144003 doi: 10.1016/j.apsusc.2019.144003 [47] Arslan M, Firat Y E, Tokg?z S R, et al. Fast electrochromic response and high coloration efficiency of Al-doped WO3 thin films for smart window applications. Ceram Int, 2021, 47(23): 32570 doi: 10.1016/j.ceramint.2021.08.152 [48] Xie Z Q, Zhang Q Q, Liu Q Q, et al. Enhanced electrochromic performance of 2D grid-structured WO3 thin films. Thin Solid Films, 2018, 653: 188 doi: 10.1016/j.tsf.2018.03.044 [49] Yuan G Z, Hua C Z, Khan S, et al. Improved electrochromic performance of WO3 films with size controlled nanorods. Electrochimica Acta, 2018, 260: 274 doi: 10.1016/j.electacta.2017.10.193 [50] Heo S, Dahlman C J, Staller C M, et al. Enhanced coloration efficiency of electrochromic tungsten oxide nanorods by site selective occupation of sodium ions. Nano Lett, 2020, 20(3): 2072 doi: 10.1021/acs.nanolett.0c00052 [51] Balaji S, Djaoued Y, Albert A S, et al. Hexagonal tungsten oxide based electrochromic devices: Spectroscopic evidence for the Li ion occupancy of four-coordinated square windows. Chem Mater, 2009, 21(7): 1381 doi: 10.1021/cm8034455 [52] Evans R C, Ellingworth A, Cashen C J, et al. Influence of single-nanoparticle electrochromic dynamics on the durability and speed of smart windows. Proc Natl Acad Sci USA, 2019, 116(26): 12666 doi: 10.1073/pnas.1822007116 [53] Guo J J, Wang M, Diao X G, et al. Prominent electrochromism achieved using aluminum ion insertion/extraction in amorphous WO3 films. J Phys Chem C, 2018, 122(33): 19037 doi: 10.1021/acs.jpcc.8b05692 [54] Zhao Q, Wang J K, Ai X H, et al. Large-area multifunctional electro-chromic-chemical device made of W17O47 nanowires by Zn2+ ion intercalation. Nano Energy, 2021, 89: 106356 doi: 10.1016/j.nanoen.2021.106356 [55] Chen X, Li W J, Wang L B, et al. Annealing effect on the electrochromic properties of amorphous WO3 films in Mg2+ based electrolytes. Mater Chem Phys, 2021, 270: 124745 doi: 10.1016/j.matchemphys.2021.124745 [56] Huo X T, Miao X W, Han X, et al. High-performance electrochromo-supercapacitors based on the synergetic effect between aqueous Al3+ and ordered hexagonal tungsten oxide nanorod arrays. J Mater Chem A, 2020, 8(19): 9927 doi: 10.1039/D0TA01808B [57] Li W J, Zhang X, Chen X, et al. Lithiation of WO3 films by evaporation method for all-solid-state electrochromic devices. Electrochimica Acta, 2020, 355: 136817 doi: 10.1016/j.electacta.2020.136817 [58] Li W J, Zhang X, Chen X, et al. Effect of independently controllable electrolyte ion content on the performance of all-solid-state electrochromic devices. Chem Eng J, 2020, 398: 125628 doi: 10.1016/j.cej.2020.125628 [59] Zhao Y M, Zhang X, Li W J, et al. High-performance electrochromic WO3 film driven by controllable crystalline structure and its all-solid-state device. Sol Energy Mater Sol Cells, 2022, 237: 111564 doi: 10.1016/j.solmat.2021.111564 [60] Jeong C Y, Kubota T, Chotsuwan C, et al. All-solid-state electrochromic device using polymer electrolytes with a wet-coated electrochromic layer. J Electroanal Chem, 2021, 897: 115614 doi: 10.1016/j.jelechem.2021.115614 [61] Shao Z W, Huang A B, Ming C, et al. All-solid-state proton-based tandem structures for fast-switching electrochromic devices. Nat Electron, 2022, 5(1): 45 doi: 10.1038/s41928-021-00697-4 [62] Jian X Y, Jin J T, Wang Y, et al. Recent progress on layered oxide cathode materials for sodium-ion batteries. Chin J Eng, 2022, 44(4): 601 doi: 10.3321/j.issn.1001-053X.2022.4.bjkjdxxb202204013菅夏琰, 金俊騰, 王瑤, 等. 鈉離子電池層狀氧化物正極材料研究進展. 工程科學學報, 2022, 44(4):601 doi: 10.3321/j.issn.1001-053X.2022.4.bjkjdxxb202204013 [63] Wang C W, Fu K, Kammampata S P, et al. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem Rev, 2020, 120(10): 4257 doi: 10.1021/acs.chemrev.9b00427 [64] Zhang S L, Li Y, Zhang T R, et al. Dual-band electrochromic devices with a transparent conductive capacitive charge-balancing anode. ACS Appl Mater Interfaces, 2019, 11(51): 48062 doi: 10.1021/acsami.9b17678 [65] Huang Q Y, Cao S, Liu Y W, et al. Boosting the Zn2+-based electrochromic properties of tungsten oxide through morphology control. Sol Energy Mater Sol Cells, 2021, 220: 110853 doi: 10.1016/j.solmat.2020.110853 [66] Chen J, Wang Z, Chen Z G, et al. Fabry-perot cavity-type electrochromic supercapacitors with exceptionally versatile color tunability. Nano Lett, 2020, 20(3): 1915 doi: 10.1021/acs.nanolett.9b05152 [67] Wu Q, Cong S, Zhao Z G. Infrared electrochromic property of the colorful tungsten oxide films. J Inorg Mater, 2021, 36(5): 485 doi: 10.15541/jim20200463武琦, 叢杉, 趙志剛. 多彩氧化鎢薄膜的紅外電致變色性能研究. 無機材料學報, 2021, 36(5):485 doi: 10.15541/jim20200463 -




 下載:
下載: