Substrate materials for high performance self-supporting oxygen catalytic electrodes
-
摘要: 在“碳達峰”和“碳中和”的時代背景下,電解水、金屬空氣電池、燃料電池等清潔能源技術由于具有能量效率高、安全性好、結構簡單和清潔環保等優點受到廣泛關注。然而,發生在氧催化電極上的關鍵反應——氧還原反應(ORR)和氧析出反應(OER)具有緩慢的動力學,很大程度上阻礙了其商業化應用。傳統氧催化電極存在合成過程繁瑣、可控性低、均一性差、成本高和載體催化劑易團聚等問題,限制了其催化性能。自支撐氧催化電極的高催化活性位點、高穩定性等優勢可以完美解決傳統電極面臨的問題。本文介紹了自支撐氧催化電極基底材料的研究進展以及合成方法,并討論了影響自支撐氧催化電極ORR/OER催化性能的因素,最后對自支撐氧催化電極未來的研發方向和發展趨勢提出展望。Abstract: Under the background of peak carbon dioxide emissions and achieving carbon neutrality, clean energy technologies such as water electrolysis, metal–air batteries, and fuel cells have attracted extensive attention due to the advantages of high efficiency, good safety, a simple structure, low cost, and eco-friendliness. However, the key reactions on oxygen catalytic electrodes, the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), are kinetically sluggish, which considerably hinders their commercial applications. The traditional oxygen catalytic electrodes with the use of binders have the disadvantages of a cumbersome synthesis process, low controllability, poor uniformity, high cost, and easy carrier catalyst agglomeration, which limit their catalytic performance. Recently, self-supported oxygen catalytic electrodes have attracted extensive attention due to their advantages of high catalytic active sites and a stabilized spatial framework, which can solve the problems faced by traditional oxygen catalytic electrodes and further improve the catalytic performance of the electrode. As the catalyst material carrier, the substrate materials play an important role in the catalytic performance of self-supporting oxygen electrodes. The high interaction forces between the substrate and the catalyst material lead to a single-direction growth orientation and uniform dispersion. Reportedly, the substrate materials for self-supporting oxygen catalytic electrodes have not been fully discussed in comprehensive reviews. Therefore, timely updates in this potential field must be provided. This paper summarizes the research progress and synthesis methods of commonly self-supporting oxygen catalytic electrodes based on different substrate materials, including two- and three-dimensional metal materials and carbon materials. In addition, this paper introduces the outstanding ORR/OER catalytic properties of common self-supporting oxygen catalytic electrodes, which are not only due to the intrinsic catalytic activity of the supported catalytic active materials but also related to the high specific surface area and high electron transfer rate caused by the structure of the self-supported electrode substrate. Finally, the future research and the development trend of self-supporting oxygen catalytic electrodes are addressed from the four aspects of density general function theory, improving electrode energy density, constructing an efficient gas–liquid–solid three-phase interface of an electrode, and establishing a standard evaluation protocol of self-supported oxygen catalytic electrodes. This review should provide new research insights for developing renewable energy storage and conversion systems.
-
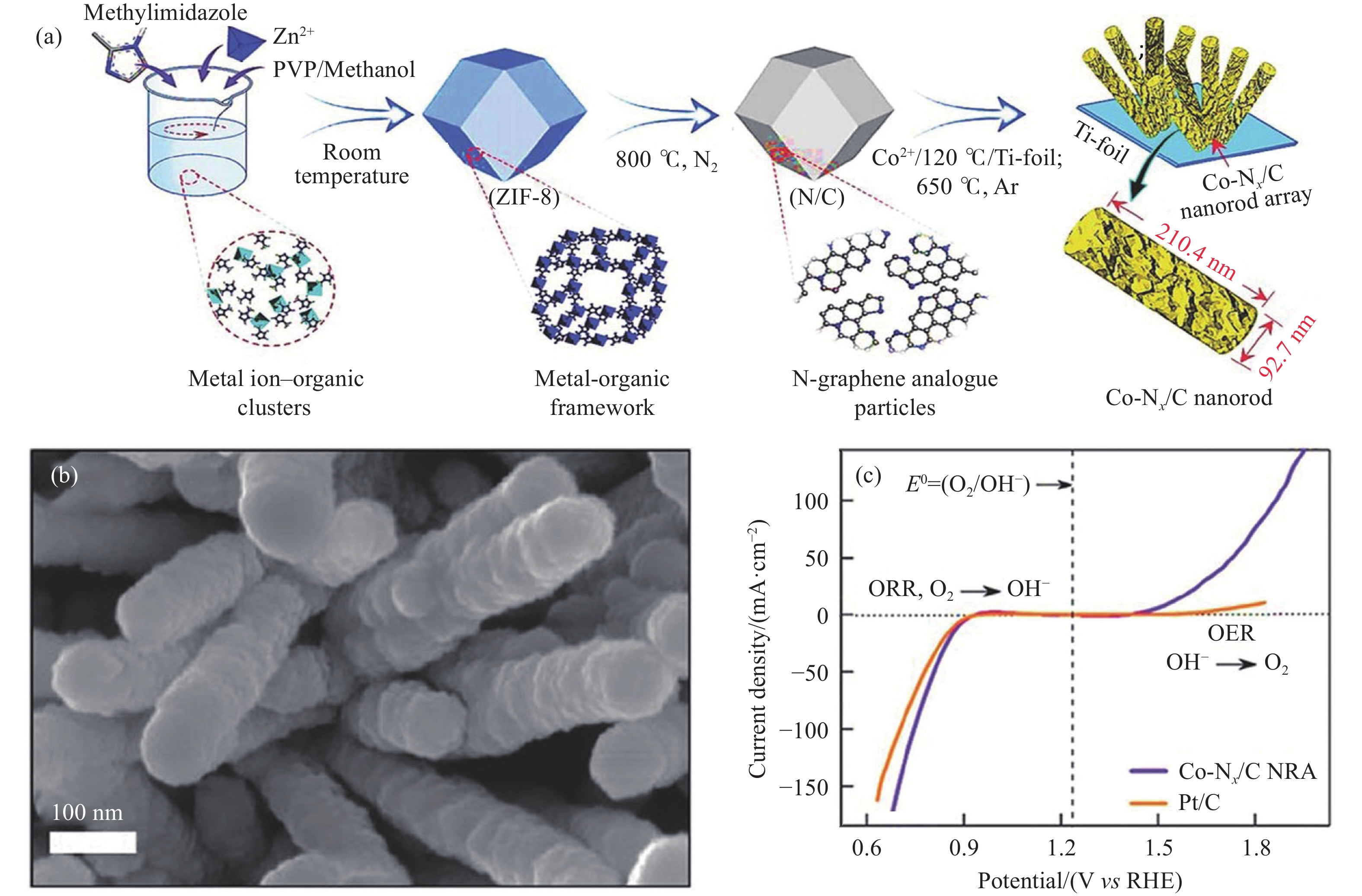
圖 1 Co–Nx/C納米棒陣列[24]. (a)合成示意圖; (b) SEM圖; (c) ORR/OER極化曲線
Figure 1. Co–Nx/C nanorod array: (a) diagrammatic scheme; (b) SEM image; (c) ORR/OER polarization curves
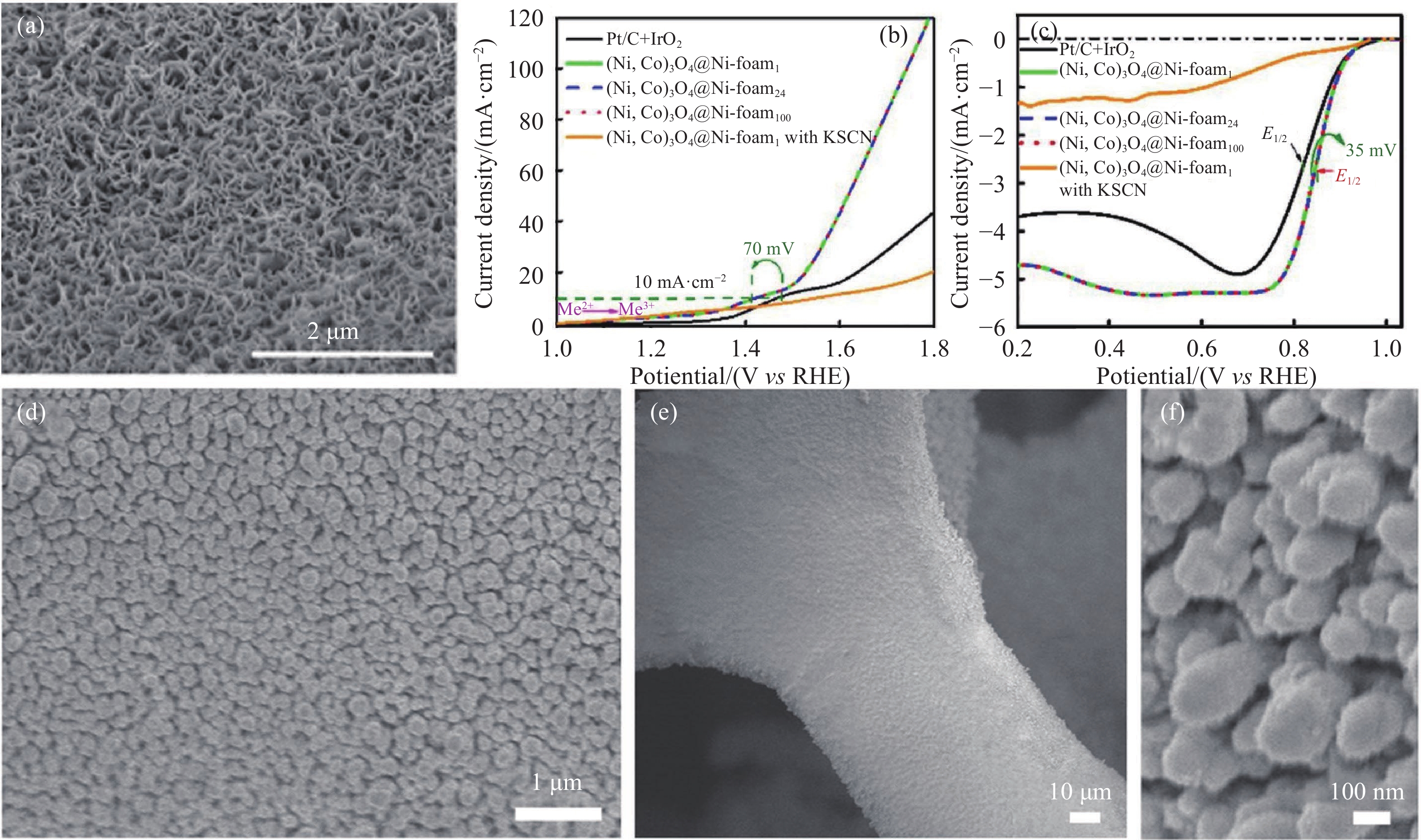
圖 2 Ni/Co氧化物催化劑及性能[35]. (a) (Ni,Co)3O4@Ni-foam24的SEM圖; (b) Pt/C+IrO2和(Ni,Co)3O4@Ni-foam電極的OER曲線; (c) Pt/C+IrO2和(Ni,Co)3O4@Ni-foam電極的ORR曲線; (d~f) CoP–MNA電極的SEM圖
Figure 2. Ni/Co catalyst and performance: (a) SEM image of (Ni,Co)3O4@Ni-foam24; (b) OER curves of Pt/C+IrO2 and (Ni,Co)3O4@Ni-foam electrodes; (c) ORR curves of Pt/C+IrO2 and (Ni,Co)3O4@Ni-foam electrodes; (d–f) SEM image of CoP–MNA electrode
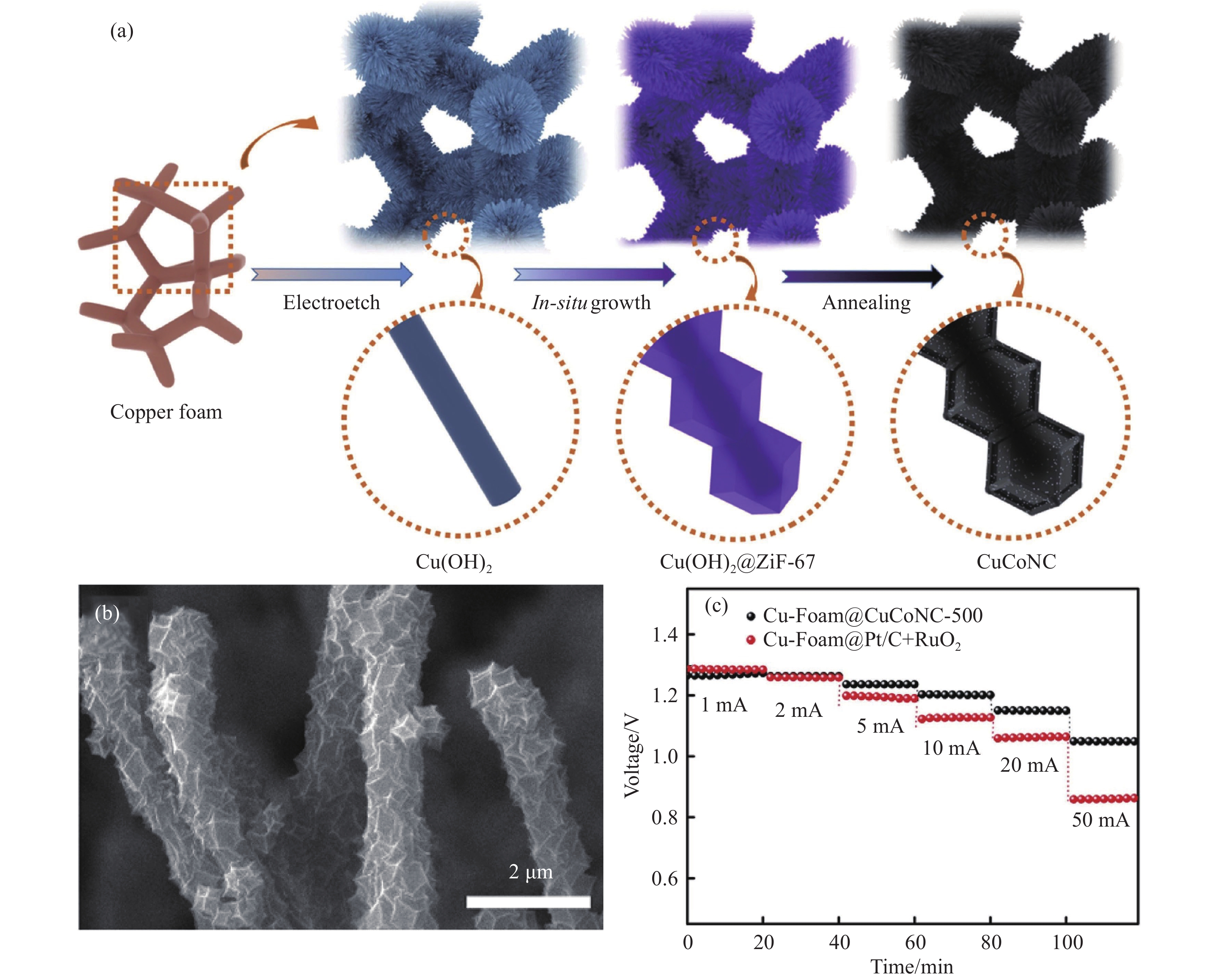
圖 4 Cu-Foam@CuCoNC-500自支撐電極及性能[48]. (a)合成示意圖; (b) SEM圖; (c) 鋅空氣電池的不同電流密度下的放電電壓圖
Figure 4. Cu-Foam@CuCoNC-500 self-supporting electrode and performance: (a) diagrammatic scheme; (b) SEM image; (c) the discharge voltage of zinc air battery under different current densities
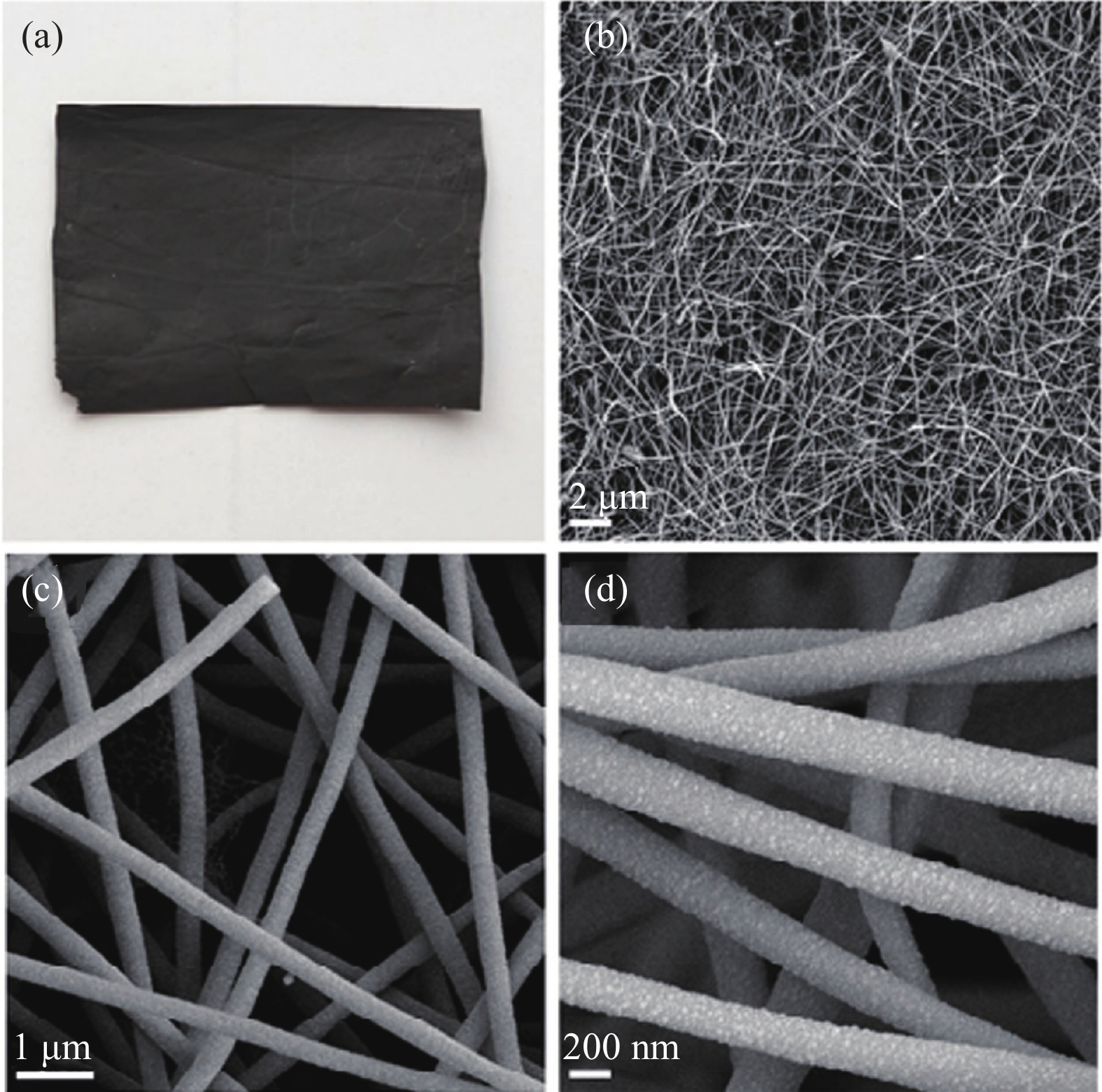
圖 5 Co/CNF電極的微觀形貌[51]. (a)光學照片; (b~d) SEM圖
Figure 5. Microstructure of Co/CNF electrode: (a) optical photo; (b–d) SEM image
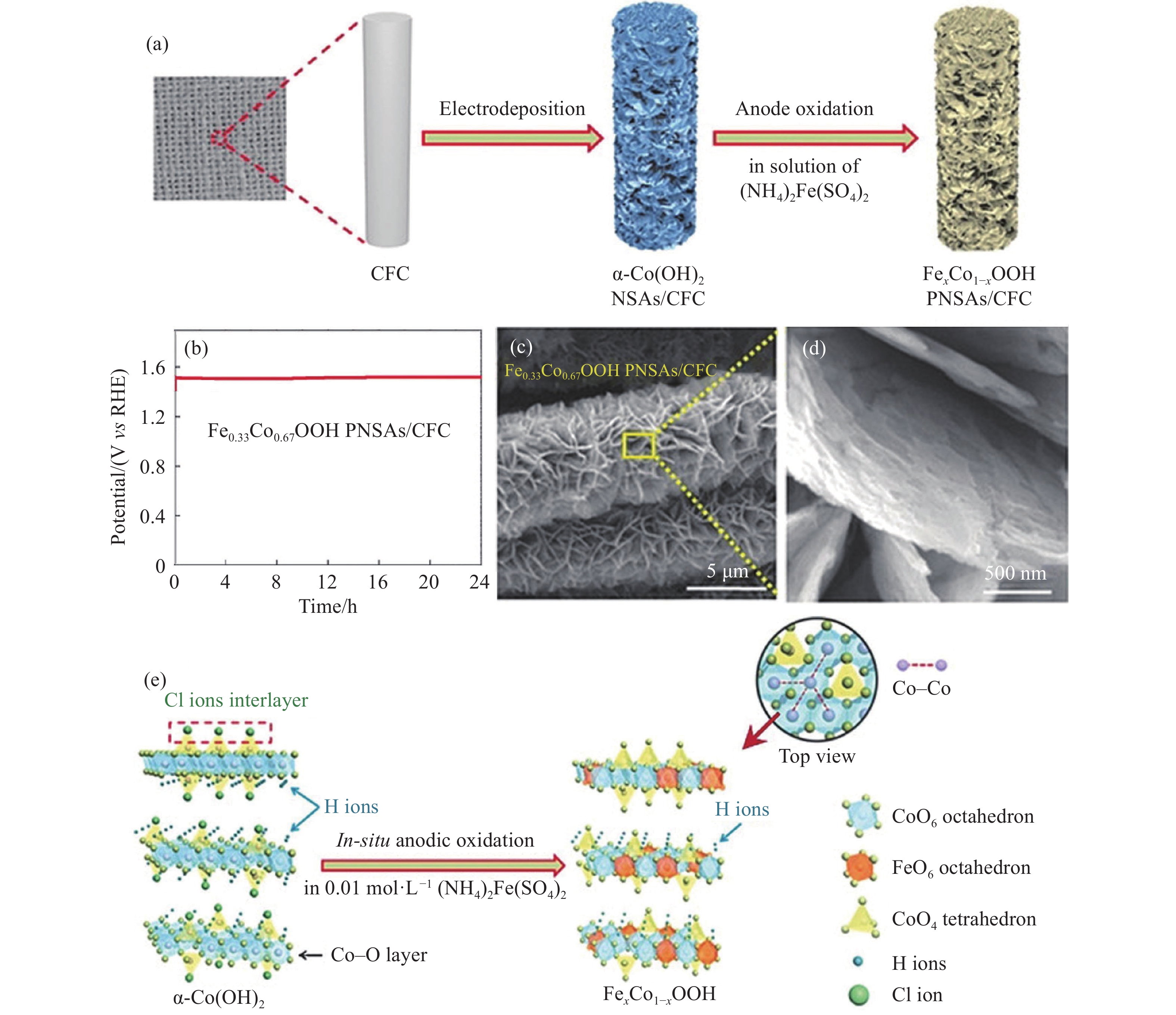
圖 7 Fe0.33Co0.67OOH PNSAs/CFC自支撐電極[56]. (a) 合成示意圖; (b) 10 mA·cm–2下的耐久性測試; (c~d) SEM圖; (e) α-Co(OH)2到Fe0.33Co0.67OOH的結構轉變示意圖
Figure 7. Fe0.33Co0.67OOH PNSAs/CFC self-supporting electrode: (a) preparation of diagram; (b) durability test at 10 mA·cm–2; (c–d) SEM images; (e) schematic diagram of structural transformation from α-Co(OH)2 to Fe0.33Co0.67OOH
表 1 泡沫鎳自支撐電極的電化學性能
Table 1. Electrochemical performance of nickel foam self-supported electrode
Electrode Electrolyte ORR OER ?E/V Full battery test Ref. E1/2/V j/(mA·cm–2) Tafel slope/
(mV·dec–1)η10 /mV Tafel slope/
(mV·dec–1)Battery Power density/
(mW·cm–2)(Ni,Co)3O4@Ni-foam electrode 1 mol·L–1 KOH 0.84 — — 180 — 0.57 Zn–air batteries 74 [35] NiFeMoS/NF–P 1 mol·L–1 KOH — 150 — 280 69 — — — [36] Ni3S2/MoSx na-nosheets/NF 1 mol·L–1 KOH — 150 — 305 67.5 — — — [37] (Ni0.33Fe0.67)2P electrode 1 mol·L–1 KOH — 50 — 230 55.9 — — — [42] CoFePO 1 mol·L–1 KOH — — — 274.5 51.7 — — — [34] CoP–MNA 1 mol·L–1 KOH — — — 290 65 — — — [19] CuCoOx/FeOOH 1 mol·L–1 KOH 0.78 5.3 — 270 63 0.72 Zn–air batteries 158 [38] Co3O4/NCNTs/3D graphene 0.1 mol·L–1 NaOH 0.80 — 66.18 — — — Al–air coin batteries 4.88 [39] NF@Co3–xNixO4 1 mol·L–1 KOH 0.91(Eonset) — — 310 — — Zn–air batteries — [40] MnO2–NiFe electrode 1 mol·L–1 KOH 1.01(Eonset) 4.26 126.1 226 251.3 0.65 Zn–air batteries 93.95 [41] MnOx–S 0.1 mol·L–1 KOH 0.95(Eonset) — 68 — 80 0.78 Zn–air batteries 69 [43] Mn–Ni3S2/NF 0.1 mol·L–1 KOH 0.347 — 107.5 — 69.3 — Zn–air batteries 75.8 [44] 表 2 鋅空氣電池的雙效碳布基自支撐電極電化學性能
Table 2. Electrochemical performance of the double-effect carbon substrate self-supported electrode for zinc–air battery
Electrode ORR OER ?E / V Ref. J /(mA·cm–2) E1/2 / V η j=10 / mV N–GQDs/NiCo2S4/CC 4.71 0.86 340 j=30 0.71 [57] Co3O4–x HoNPs@HPNCS-60 5.82 0.83 313 0.74 [58](2019a) NC–Co3O4/CC-600 — — 210 0.87 [59] SS–Co–SAC NSAs 59.1 0.81 348 0.77 [60] Co4N/CNW/CC 16.5 0.80 310 0.74 [61] FeNO–CNT–CNFF-800 — 0.87 — 0.79 [62] Co–FeCo/N–G 2.28 0.82 258 — [63] NPC/Fe–N–C 5.2 0.87 — — [64] www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Gür T M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ Sci, 2018, 11(10): 2696 doi: 10.1039/C8EE01419A [2] Li J C, Yang Z Q, Tang D M, et al. N-doped carbon nanotubes containing a high concentration of single iron atoms for efficient oxygen reduction. NPG Asia Mater, 2018, 10(1): e461 doi: 10.1038/am.2017.212 [3] Wang Z, Ang J M, Zhang B W, et al. FeCo/FeCoNi/N-doped carbon nanotubes grafted polyhedron-derived hybrid fibers as bifunctional oxygen electrocatalysts for durable rechargeable zinc–air battery. Appl Catal B Environ, 2019, 254: 26 doi: 10.1016/j.apcatb.2019.04.027 [4] Mandal M. Recent advancement on anion exchange membranes for fuel cell and water electrolysis. ChemElectroChem, 2021, 8(1): 36 doi: 10.1002/celc.202001329 [5] Zhao H W, Xing T Y, Li L X, et al. Synthesis of cobalt and nitrogen co-doped carbon nanotubes and its ORR activity as the catalyst used in hydrogen fuel cells. Int J Hydrog Energy, 2019, 44(46): 25180 doi: 10.1016/j.ijhydene.2019.03.271 [6] Wei C, Rao R R, Peng J Y, et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv Mater, 2019, 31(31): 1806296 doi: 10.1002/adma.201806296 [7] Gao Y Y, Wang L, Li G Z, et al. Taming transition metals on N-doped CNTs by a one-pot method for efficient oxygen reduction reaction. Int J Hydrog Energy, 2018, 43(16): 7893 doi: 10.1016/j.ijhydene.2018.03.043 [8] Wang L P, Tian J, Li J S, et al. Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction. J Cent South Univ, 2019, 26(6): 1458 doi: 10.1007/s11771-019-4102-y [9] Zhao Y M, Yu G Q, Wang F F, et al. Bioinspired transition-metal complexes as electrocatalysts for the oxygen reduction reaction. Chem Eur J, 2019, 25(15): 3726 doi: 10.1002/chem.201803764 [10] Liu K, Huang X B, Wang H Y, et al. Co3O4–CeO2/C as a highly active electrocatalyst for oxygen reduction reaction in Al-air batteries. ACS Appl Mater Interfaces, 2016, 8(50): 34422 doi: 10.1021/acsami.6b12294 [11] Li F Z, Fu L, Li J S, et al. Fe7C3–Fe3N/FeNxCy decorated carbon material as highly efficient catalyst for oxygen reduction reaction in Al–air batteries. Nanosci Nanotechnol Lett, 2017, 9(12): 1909 doi: 10.1166/nnl.2017.2547 [12] Wang H, Chen H Z, Wang H J, et al. Hierarchical porous FeCo2O4@Ni as a carbon- and binder-free cathode for lithium–oxygen batteries. J Alloys Compd, 2019, 780: 107 doi: 10.1016/j.jallcom.2018.11.231 [13] Song L, Tang J, Wang T, et al. Self-supported ZIF-derived Co3O4 nanoparticles-decorated porous N-doped carbon fibers as oxygen reduction catalyst. Chem Eur J, 2019, 25(27): 6807 doi: 10.1002/chem.201900197 [14] Wang P C, Wan L, Xu Z A, et al. Interface engineering of self-supported electrode for electrochemical water splitting. Energy Storage Sci Technol, 2022, 11(6): 1934 doi: 10.19799/j.cnki.2095-4239.2022.0195王培燦, 萬磊, 徐子昂, 等. 基于界面工程的自支撐催化電極用于電解水制氫. 儲能科學與技術, 2022, 11(6):1934 doi: 10.19799/j.cnki.2095-4239.2022.0195 [15] Jung J W, Cho S H, Nam J S, et al. Current and future cathode materials for non-aqueous Li–air (O2) battery technology: A focused review. Energy Storage Mater, 2020, 24: 512 doi: 10.1016/j.ensm.2019.07.006 [16] Tang C, Wang H F, Zhang Q. Multiscale principles to boost reactivity in gas-involving energy electrocatalysis. Acc Chem Res, 2018, 51(4): 881 doi: 10.1021/acs.accounts.7b00616 [17] Wang Y, Fu J, Zhang Y N, et al. Continuous fabrication of a MnS/Co nanofibrous air electrode for wide integration of rechargeable zinc–air batteries. Nanoscale, 2017, 9(41): 15865 doi: 10.1039/C7NR05728H [18] Li Z H, Shao M F, Yang Q H, et al. Directed synthesis of carbon nanotube arrays based on layered double hydroxides toward highly-efficient bifunctional oxygen electrocatalysis. Nano Energy, 2017, 37: 98 doi: 10.1016/j.nanoen.2017.05.016 [19] Zhu Y P, Liu Y P, Ren T Z, et al. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv Funct Mater, 2015, 25(47): 7337 doi: 10.1002/adfm.201503666 [20] Ma T Y, Dai S, Qiao S Z. Self-supported electrocatalysts for advanced energy conversion processes. Mater Today, 2016, 19(5): 265 doi: 10.1016/j.mattod.2015.10.012 [21] Xie L S, Li X L, Wang B, et al. Molecular engineering of a 3D self-supported electrode for oxygen electrocatalysis in neutral media. Angew Chem Int Ed Engl, 2019, 58(52): 18883 doi: 10.1002/anie.201911441 [22] Zhang X Y, Kang J L. Research progress of flexible self-supporting nanostructure electrodes. Mater Rep, 2020, 34(Suppl 2): 1030張曦元, 康建立. 柔性自支撐納米結構電極的研究進展. 材料導報, 2020, 34(增刊 2):1030 [23] Niu Y L, Teng X, Gong S Q, et al. A bimetallic alloy anchored on biomass-derived porous N-doped carbon fibers as a self-supporting bifunctional oxygen electrocatalyst for flexible Zn–air batteries. J Mater Chem A, 2020, 8(27): 13725 doi: 10.1039/D0TA03288C [24] Amiinu I S, Liu X B, Pu Z H, et al. From 3D ZIF nanocrystals to Co–Nx/C nanorod array electrocatalysts for ORR, OER, and Zn–air batteries. Adv Funct Mater, 2018, 28(5): 1704638 doi: 10.1002/adfm.201704638 [25] Yang C H, Makabu C M, Du X H, et al. Cobalt nanorods decorated titanium oxide arrays as efficient and stable electrocatalyst for oxygen evolution reaction. Electrochimica Acta, 2021, 396: 139213 doi: 10.1016/j.electacta.2021.139213 [26] Lv Q, Wang N, Si W Y, et al. Pyridinic nitrogen exclusively doped carbon materials as efficient oxygen reduction electrocatalysts for Zn–air batteries. Appl Catal B Environ, 2020, 261: 118234 doi: 10.1016/j.apcatb.2019.118234 [27] Zhang C F, Chen Z W, Lian Y B, et al. Copper-based conductive metal organic framework in-situ grown on copper foam as a bifunctional electrocatalyst. Acta Phys Chimica Sin, 2019, 35(12): 1404 doi: 10.3866/PKU.WHXB201905030張楚風, 陳哲偉, 連躍彬, 等. 泡沫銅基底原位生長的銅基導電金屬有機框架作為雙功能電催化劑. 物理化學學報, 2019, 35(12):1404 doi: 10.3866/PKU.WHXB201905030 [28] Ma T Y, Dai S, Jaroniec M, et al. Metal-organic framework derived hybrid Co3O4–carbon porous nanowire arrays as reversible oxygen evolution electrodes. J Am Chem Soc, 2014, 136(39): 13925 doi: 10.1021/ja5082553 [29] Cai G R, Zhang W, Jiao L, et al. Template-directed growth of well-aligned MOF arrays and derived self-supporting electrodes for water splitting. Chem, 2017, 2(6): 791 doi: 10.1016/j.chempr.2017.04.016 [30] Jiang J, Li Y Y, Liu J P, et al. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater, 2012, 24(38): 5166 doi: 10.1002/adma.201202146 [31] Qazi U Y, Yuan C Z, Ullah N, et al. One-step growth of iron-nickel bimetallic nanoparticles on FeNi alloy foils: Highly efficient advanced electrodes for the oxygen evolution reaction. ACS Appl Mater Interfaces, 2017, 9(34): 28627 doi: 10.1021/acsami.7b08922 [32] Yuan C Z, Sun Z T, Jiang Y F, et al. One-step in situ growth of iron-nickel sulfide nanosheets on FeNi alloy foils: High-performance and self-supported electrodes for water oxidation. Small, 2017, 13(18): 1604161 doi: 10.1002/smll.201604161 [33] Wang P C, Wang R Z, Xu Q, et al. Role of the interfacial effect between the substrate and Co(OH)2 layer in electrochemical oxygen evolution. ACS Appl Energy Mater, 2021, 4(9): 9487 doi: 10.1021/acsaem.1c01671 [34] Duan J J, Chen S, Vasileff A, et al. Anion and cation modulation in metal compounds for bifunctional overall water splitting. ACS Nano, 2016, 10(9): 8738 doi: 10.1021/acsnano.6b04252 [35] Xu N N, Wilson J A, Wang Y D, et al. Flexible self-supported bi-metal electrode as a highly stable carbon- and binder-free cathode for large-scale solid-state zinc–air batteries. Appl Catal B Environ, 2020, 272: 118953 doi: 10.1016/j.apcatb.2020.118953 [36] Yan K L, Qin J F, Liu Z Z, et al. Organic–inorganic hybrids-directed ternary NiFeMoS anemone-like nanorods with scaly surface supported on nickel foam for efficient overall water splitting. Chem Eng J, 2018, 334: 922 doi: 10.1016/j.cej.2017.10.074 [37] Xu Y Z, Yuan C Z, Chen X P. One-pot synthesis nickel sulfide/amorphous molybdenum sulfide nanosheets array on nickel foam as a robust oxygen evolution reaction electrocatalyst. J Solid State Chem, 2017, 256: 124 doi: 10.1016/j.jssc.2017.08.014 [38] Kuang M, Wang Q H, Ge H T, et al. CuCoOx/FeOOH core–shell nanowires as an efficient bifunctional oxygen evolution and reduction catalyst. ACS Energy Lett, 2017, 2(10): 2498 doi: 10.1021/acsenergylett.7b00835 [39] Liu Y S, Yang L S, Xie B, et al. Ultrathin Co3O4 nanosheet clusters anchored on nitrogen doped carbon nanotubes/3D graphene as binder-free cathodes for Al–air battery. Chem Eng J, 2020, 381: 122681 doi: 10.1016/j.cej.2019.122681 [40] Wu M J, Zhang G X, Chen N, et al. A self-supported electrode as a high-performance binder- and carbon-free cathode for rechargeable hybrid zinc batteries. Energy Storage Mater, 2020, 24: 272 doi: 10.1016/j.ensm.2019.08.009 [41] Wang P C, Lin Y Q, Wan L, et al. Construction of a Janus MnO2–NiFe electrode via selective electrodeposition strategy as a high-performance bifunctional electrocatalyst for rechargeable zinc–air batteries. ACS Appl Mater Interfaces, 2019, 11(41): 37701 doi: 10.1021/acsami.9b12232 [42] Li Y J, Zhang H C, Jiang M, et al. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting. Adv Funct Mater, 2017, 27(37): 1702513 doi: 10.1002/adfm.201702513 [43] Radenahmad N, Khezri R, Mohamad A A, et al. A durable rechargeable zinc–air battery via self-supported MnOx–S air electrode. J Alloys Compd, 2021, 883: 160935 doi: 10.1016/j.jallcom.2021.160935 [44] Yin M M, Miao H, Chen B, et al. Self-supported metal sulfide electrode for flexible quasi-solid-state zinc–air batteries. J Alloys Compd, 2021, 878: 160434 doi: 10.1016/j.jallcom.2021.160434 [45] Li X Y, Qian X, Xu Y L, et al. Electrodeposited cobalt phosphides with hierarchical nanostructure on biomass carbon for bifunctional water splitting in alkaline solution. J Alloys Compd, 2020, 829: 154535 doi: 10.1016/j.jallcom.2020.154535 [46] Wang P C, Jia T, Wang B G. Review–recent advance in self-supported electrocatalysts for rechargeable zinc–air batteries. J Electrochem Soc, 2020, 167(11): 110564 doi: 10.1149/1945-7111/aba96e [47] Wang P C, Lin Y Q, Xu Q, et al. Acid-corrosion-induced hollow-structured NiFe-layered double hydroxide electrocatalysts for efficient water oxidation. ACS Appl Energy Mater, 2021, 4(9): 9022 doi: 10.1021/acsaem.1c01314 [48] Sun H, Li Q, Lian Y B, et al. Highly efficient water splitting driven by zinc–air batteries with a single catalyst incorporating rich active species. Appl Catal B Environ, 2020, 263: 118139 doi: 10.1016/j.apcatb.2019.118139 [49] Wang L P, Fu L, Li J S, et al. On an easy way to prepare highly efficient Fe/N-co-doped carbon nanotube/nanoparticle composite for oxygen reduction reaction in Al–air batteries. J Mater Sci, 2018, 53(14): 10280 doi: 10.1007/s10853-018-2245-0 [50] He S J, Chen W. Progresses of self-supported supercapacitor electrode materials based on carbon substrates. J Electrochem, 2015, 21(6): 518 doi: 10.13208/j.electrochem.150843何水劍, 陳衛. 碳基三維自支撐超級電容器電極材料研究進展. 電化學, 2015, 21(6):518 doi: 10.13208/j.electrochem.150843 [51] Zhang X, Zhou F L, Zhang X X, et al. Carbon fibers loaded with Co nanoparticles prepared by electrospinning as self-supporting cathode materials for Zn–air batteries. J Nat Sci Heilongjiang Univ, 2022, 39(3): 329張旭, 周方玲, 張欣欣, 等. 靜電紡絲制備Co/碳纖維自支撐膜用于鋅-空電池正極. 黑龍江大學自然科學學報, 2022, 39(3):329 [52] Zeng S, Lv B, Qiao J, et al. PtFe alloy nanoparticles confined on carbon nanotube networks as air cathodes for flexible and wearable energy devices. ACS Appl Nano Mater, 2019, 2(12): 7870 doi: 10.1021/acsanm.9b01865 [53] Lu W L, Jiang R, Yin X, et al. Porous N-doped-carbon coated CoSe2 anchored on carbon cloth as 3D photocathode for dye-sensitized solar cell with efficiency and stability outperforming Pt. Nano Res, 2019, 12(1): 159 doi: 10.1007/s12274-018-2195-5 [54] Wang X Q, He J R, Yu B, et al. CoSe2 nanoparticles embedded MOF-derived Co–N–C nanoflake arrays as efficient and stable electrocatalyst for hydrogen evolution reaction. Appl Catal B Environ, 2019, 258: 117996 doi: 10.1016/j.apcatb.2019.117996 [55] Peng L W, Wang Y X, Masood I, et al. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction. Appl Catal B Environ, 2020, 264: 118447 doi: 10.1016/j.apcatb.2019.118447 [56] Ye S H, Shi Z X, Feng J X, et al. Activating CoOOH porous nanosheet arrays by partial iron substitution for efficient oxygen evolution reaction. Angew Chem Int Ed, 2018, 57(10): 2672 doi: 10.1002/anie.201712549 [57] Liu W W, Ren B H, Zhang W Y, et al. Defect-enriched nitrogen doped-graphene quantum dots engineered NiCo2S4 nanoarray as high-efficiency bifunctional catalyst for flexible Zn–air battery. Small, 2019, 15(44): e1903610 doi: 10.1002/smll.201903610 [58] Ji D X, Fan L, Tao L, et al. The kirkendall effect for engineering oxygen vacancy of hollow Co3O4 nanoparticles toward high-performance portable zinc–air batteries. Angew Chem Int Ed, 2019, 58(39): 13840 doi: 10.1002/anie.201908736 [59] Liu Q, Wang L, Liu X, et al. N-doped carbon-coated Co3O4 nanosheet array/carbon cloth for stable rechargeable Zn–air batteries. Sci China Mater, 2019, 62(5): 624 doi: 10.1007/s40843-018-9359-7 [60] Xie W F, Song Y K, Li S J, et al. Single-atomic-Co electrocatalysts with self-supported architecture toward oxygen-involved reaction. Adv Funct Mater, 2019, 29(50): 1906477 doi: 10.1002/adfm.201906477 [61] Meng F L, Zhong H X, Bao D, et al. In situ coupling of strung Co4N and intertwined N–C fibers toward free-standing bifunctional cathode for robust, efficient, and flexible Zn–air batteries. J Am Chem Soc, 2016, 138(32): 10226 doi: 10.1021/jacs.6b05046 [62] Ji D X, Peng S J, Safanama D, et al. Design of 3-dimensional hierarchical architectures of carbon and highly active transition metals (Fe, Co, Ni) as bifunctional oxygen catalysts for hybrid lithium–air batteries. Chem Mater, 2017, 29(4): 1665 doi: 10.1021/acs.chemmater.6b05056 [63] Jin Q Y, Ren B W, Chen J P, et al. A facile method to conduct 3D self-supporting Co–FeCo/N-doped graphene-like carbon bifunctional electrocatalysts for flexible solid-state zinc air battery. Appl Catal B Environ, 2019, 256: 117887 doi: 10.1016/j.apcatb.2019.117887 [64] Zhang G Y, Liu X, Yu P, et al. Fe3C coupled with Fe–Nx supported on N-doped carbon as oxygen reduction catalyst for assembling Zn–air battery to drive water splitting. Chin Chem Lett, 2022, 33(8): 3903 doi: 10.1016/j.cclet.2021.11.075 [65] Wang P C, Wang B G. C–O–Co bond-stabilized CoP on carbon cloth toward hydrogen evolution reaction. Int J Hydrog Energy, 2022, 47(15): 9209 doi: 10.1016/j.ijhydene.2021.12.264 [66] Cheng Y H, Li D M, Shi L, et al. Efficient unitary oxygen electrode for air-based flow batteries. Nano Energy, 2018, 47: 361 doi: 10.1016/j.nanoen.2018.03.013 [67] Kosimaningrum W E, Le T X H, Holade Y, et al. Surfactant- and binder-free hierarchical platinum nanoarrays directly grown onto a carbon felt electrode for efficient electrocatalysis. ACS Appl Mater Interfaces, 2017, 9(27): 22476 doi: 10.1021/acsami.7b04651 [68] Li R Q, Wang B L, Gao T, et al. Monolithic electrode integrated of ultrathin NiFeP on 3D strutted graphene for bifunctionally efficient overall water splitting. Nano Energy, 2019, 58: 870 doi: 10.1016/j.nanoen.2019.02.024 [69] Jiang L L, Wang Y Q, Lu Y. Recent research on flexible, free-standing graphene-based electrodes for supercapacitors. J Xihua Univ, 2020, 39(3): 97 doi: 10.12198/j.issn.1673159X.3675姜麗麗, 王雅琴, 魯云. 柔性自支撐石墨烯基復合超級電容器電極材料研究進展. 西華大學學報(自然科學版), 2020, 39(3):97 doi: 10.12198/j.issn.1673159X.3675 [70] Zhao Y G, Li L, Liu D K, et al. Sponge tofu-like graphene-carbon hybrid supporting Pt–Co nanocrystals for efficient oxygen reduction reaction and Zn–Air battery. Int J Hydrog Energy, 2021, 46(29): 15561 doi: 10.1016/j.ijhydene.2021.02.126 -




 下載:
下載: