Research progress of hydrophobic modification of silica aerogel for oil spill pollution treatment
-
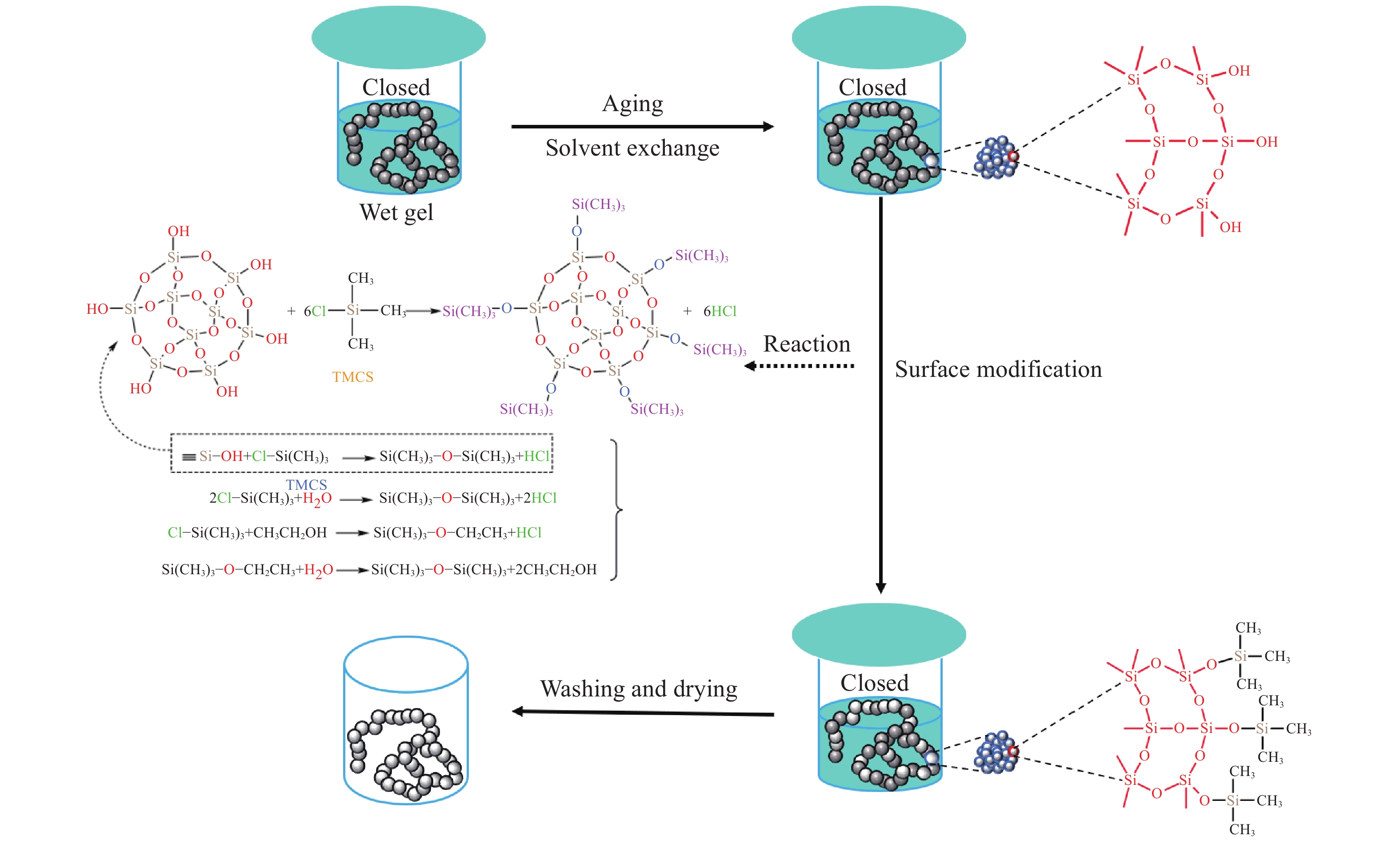
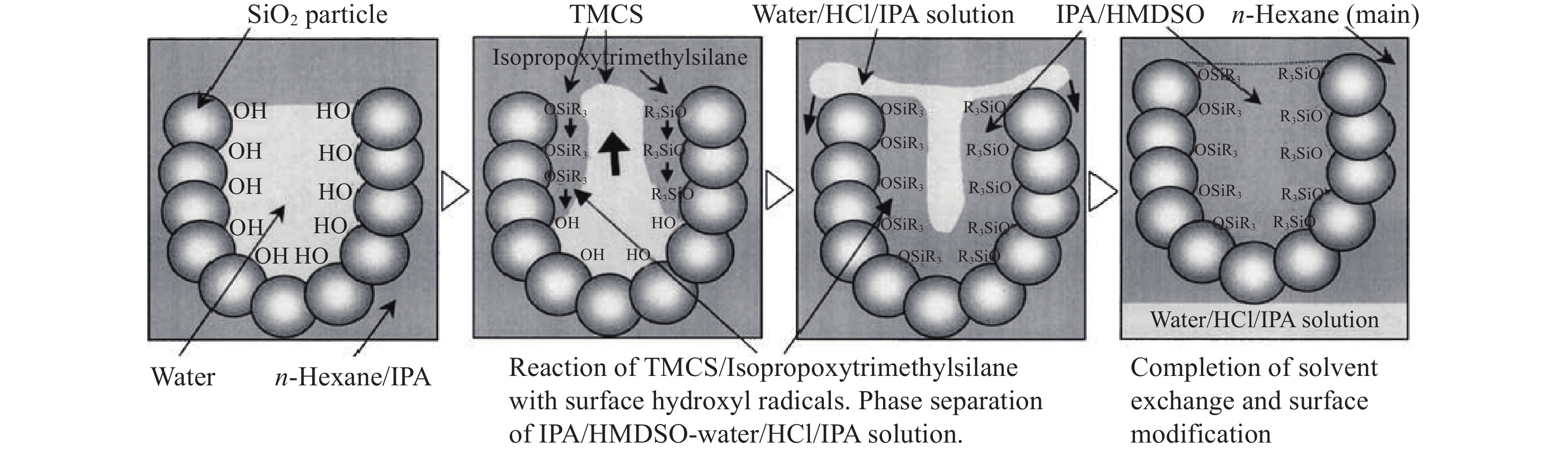
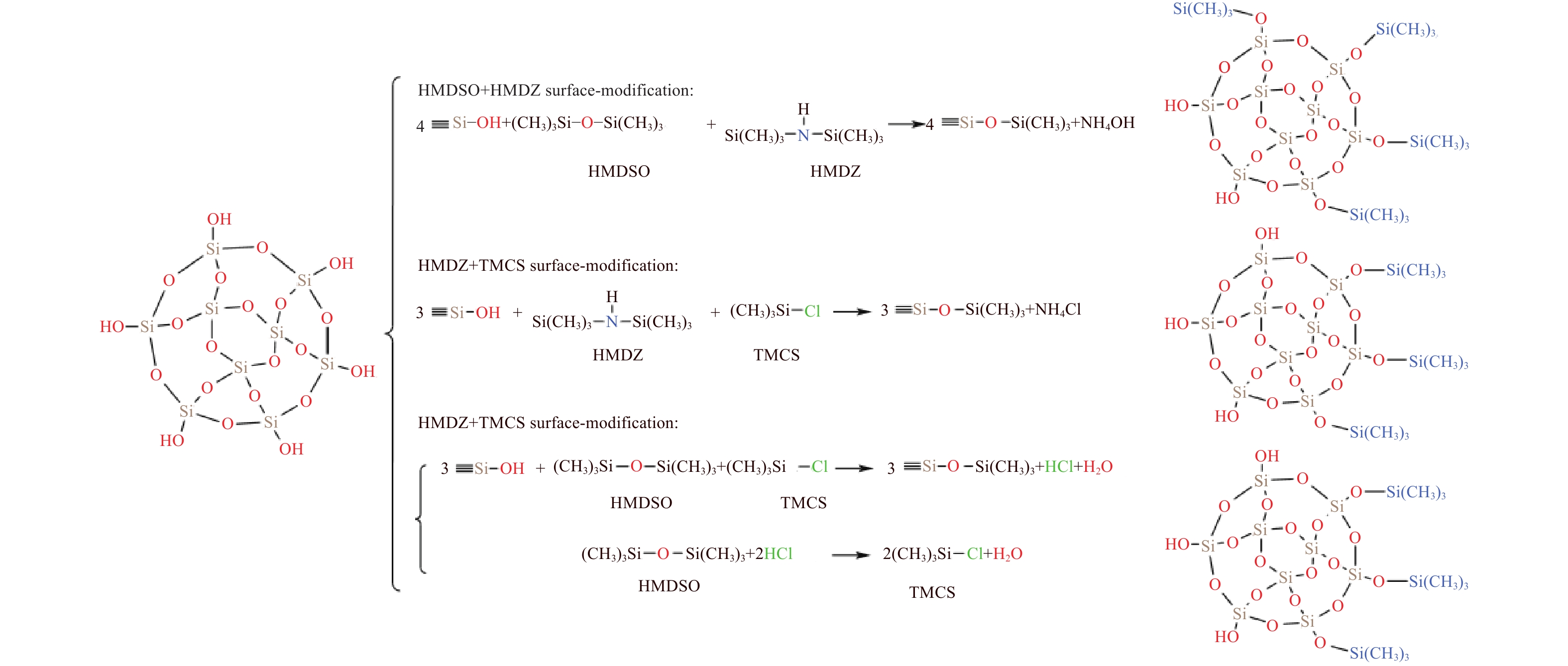
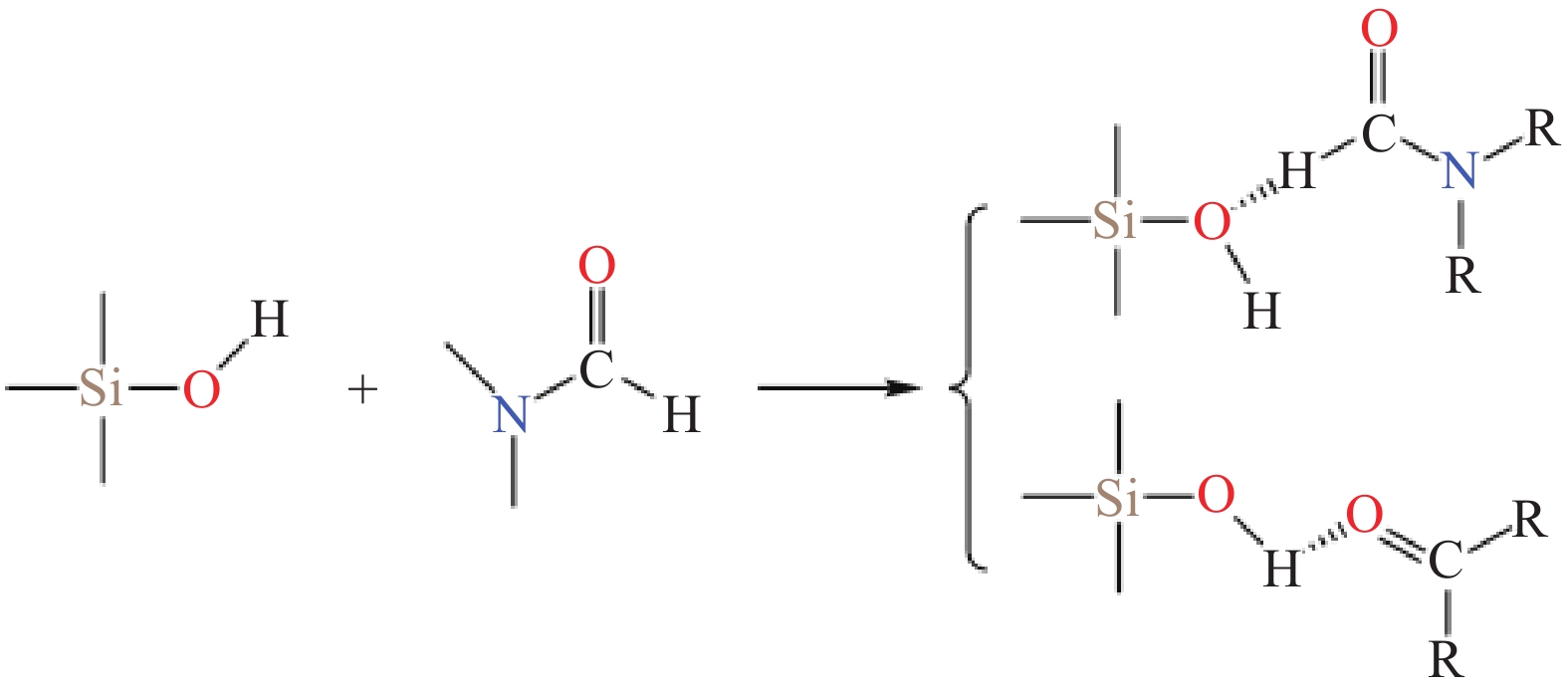
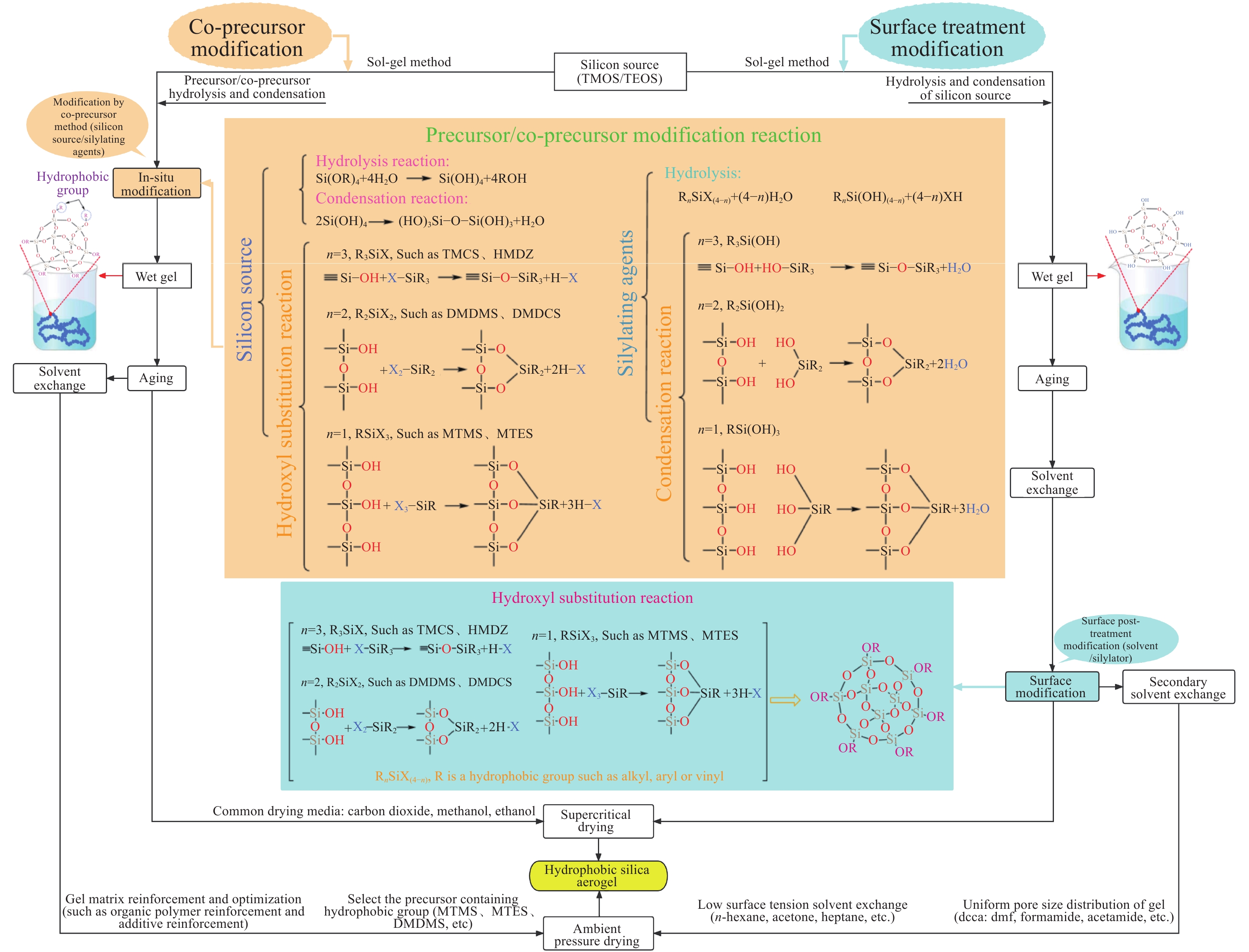
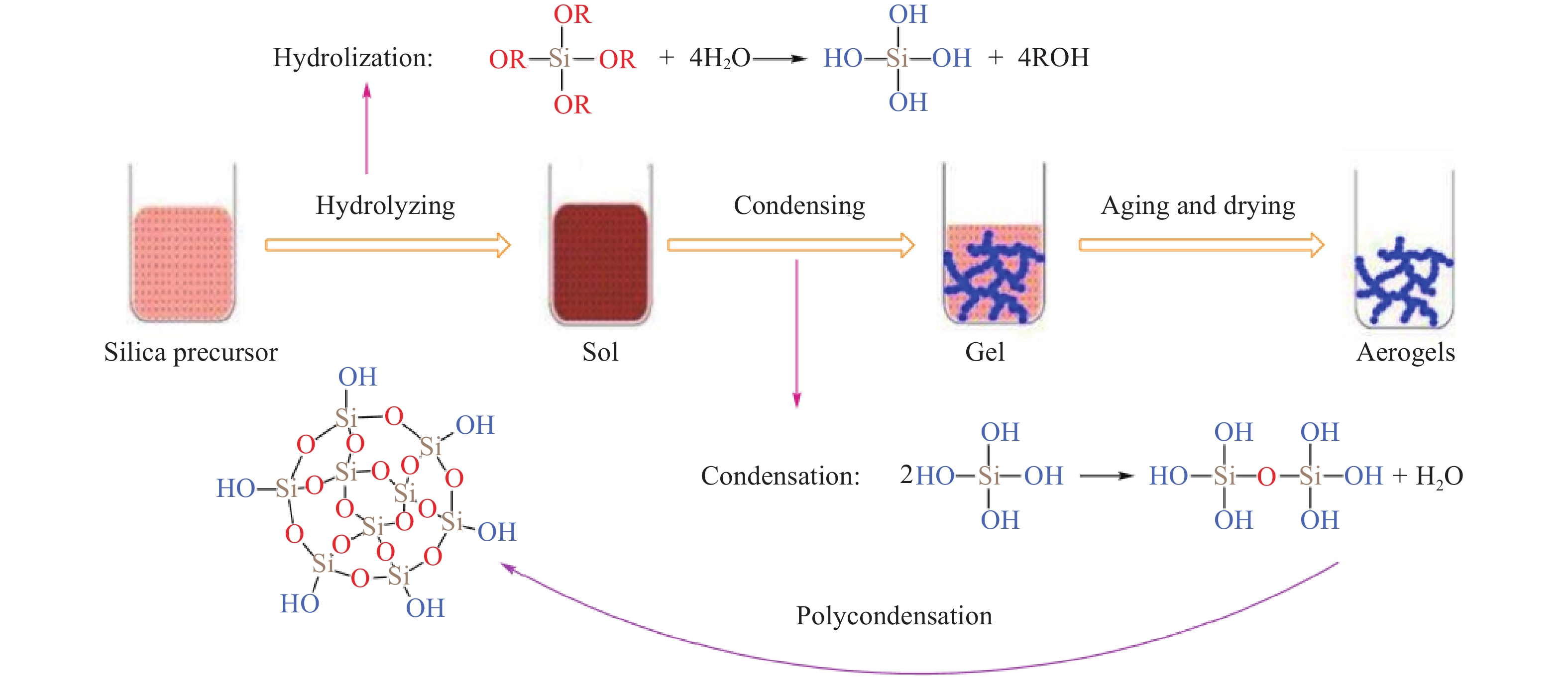
摘要: 二氧化硅氣凝膠(Silica aerogel,SA)具有高孔隙率、低密度、高比表面積等特性,可成為一種良好的吸油材料,然而親水表面和珍珠項鏈的結構限制了其在吸油領域的廣泛應用。疏水改性后的疏水SiO2氣凝膠(Hydrophobic silica aerogel,HSA)不僅具有SA的優異特性,而且疏水/親油性好,是一種優異的輕質吸油材料。本文以表面后處理法和共前驅體法制備HSA為主線,系統介紹了這兩種方法結合超臨界干燥和常壓干燥制備HSA的研究進展,分析總結了兩種方法的優缺點。其中,共前驅體法主要結合超臨界干燥工藝制備HSA,表面后處理法則常結合常壓干燥,兩種方法主要都采用硅烷化劑為疏水改性劑。表面后處理法改性不改變已形成的孔隙結構,HSA的孔徑和粒徑比較均勻,但可能存在內部改性不徹底的問題。共前驅體法在凝膠結構形成的同時完成改性,制備的HSA比表面積更大,疏水性更好,但是其孔徑不均勻,引入的疏水基團有限。此外,本文還綜述了目前常用的提高HSA機械性能的方法以及HSA吸油性能的研究進展。最后,立足于當前HSA用作吸油材料發展的趨勢,對HSA吸油材料朝著開發低成本且環境友好的原料、開發周期短的疏水改性流程、制備大塊體HSA、提高HSA的機械性能以及提高其吸油性能等發展方向進行了展望。Abstract: Oil spill pollution seriously endangers human and ecosystem health. Therefore, it is urgent to develop oil-absorbing materials to effectively remove oil spill pollution. Among the traditional oil-absorbing materials, natural organic adsorption material has low oil absorption capacity and hydrophilicity; inorganic adsorption materials are difficult to recover and have low oil absorption efficiency and high price; and although the synthetic organic adsorbent has outstanding oil absorption capacity, its biodegradation is poor. Silica aerogel (SA) has the characteristics of high porosity, low density, and high specific surface area, which make it an excellent oil-absorbing material. However, the hydrophilic surface and pearl necklace structure of SA limit its wide applications in the oil absorption field. Hydrophobically modified hydrophobic silica aerogel (HSA) has not only excellent SA characteristics but also good hydrophobic/lipophilic properties. In this paper, focusing on HSA preparation by surface posttreatment modification and coprecursor modification, the research progress on these two methods combined with supercritical drying and ambient pressure drying is systematically introduced, and the advantages and disadvantages of the two methods are analyzed and summarized. The coprecursor modification is mainly combined with a supercritical drying process to prepare HSA, while the surface posttreatment modification is often combined with an ambient pressure drying process. Both methods normally use silylating agents as hydrophobic modifiers. The surface posttreatment modification does not change the formed pore structure, and the pore size and particle size of HSA are relatively uniform. However, the modification process of surface posttreatment is long, the solvent consumption is large, and the cost is high. In addition, incomplete internal modification may be a problem. In the coprecursor modification method, wet gel is formed and modified simultaneously, shortening the modification time and saving costs. The prepared HSA of coprecursor modification has a larger specific surface area and better hydrophobicity, but its pore size is uneven, and the introduced hydrophobic groups are limited. Excessive silylating agents affect the sol–gel process of HSA. In addition, the current methods for strengthening HSA mechanical properties and the research progress on HSA oil absorption properties are reviewed. Finally, based on the current development of HSA as oil-absorbing materials, the development direction of these materials is discussed, for example, developing low-cost and eco-friendly raw materials, shortening the hydrophobic modification process, preparing bulk HSA, strengthening the mechanical properties, and improving the oil-absorbing properties of HSA.
-
表 文中所用到的化合物縮寫名稱表
Table . List of abbreviated names of compounds used in this paper
Full name Abbreviation Full name Abbreviation Tetramethoxysilane (正硅酸甲酯) TMOS Polydiethyloxysiloxane (聚二乙氧基硅氧烷) PDEOS Tetraethoxysilane (正硅酸四乙酯) TEOS Isopropanol (異丙醇) IPA Dimethyldichlorosilane (二甲基二氯硅烷) DMDCS Phenyltriethoxysilane (苯基三乙氧基硅烷) PTES Dimethylchlorosilane (二甲基氯硅烷) DMCS Hexamethyldisilazane (六甲基二硅氮烷) HMDZ Trimethylchlorosilane (三甲基氯硅烷) TMCS Hexamethyldisiloxane (六甲基二硅氧烷) HMDSO Trimethylmethoxysilane (三甲基甲氧基硅烷) TMMS N. N-dimethylformamide (N,N-二甲基甲酰胺) DMF Trimethylethoxysilane (三甲基乙氧基硅烷) TMES 3-(trimethoxysilylpropyl) methacrylate
[3-(三甲氧基硅基丙基)甲基丙烯酸酯]TMSPM Methyltriethoxysilane (甲基三乙氧基硅烷) MTES 2,5-divinyltrimethoxysilane (2,5-二乙烯基三甲氧基硅烷) DVTHP Methyltrimethoxysilane (甲基三甲氧基硅烷) MTMS 3-methylpropenyloxypropyltrimethoxysilane
(3-甲基丙烯氧基丙基三甲氧基硅烷)MEMO Ethyltrimethoxysilane (乙基三甲氧基硅烷) ETMS 3-glycidyloxypropyltrimethoxysilane
(3-縮水甘油氧基丙基三甲氧基硅烷)GLYMO Ethyltriethoxysilane (乙基三乙氧基硅烷) ETES Dodecyltrimethoxysilane (十二烷基三甲氧基硅烷) DTMS Propyltrimethoxysilane (丙基三甲氧基硅烷) PTMS Type Structure R/Si RnSiX4–n Mono-functional 
3 Trimethylchlorosilane (TMCS), Hexamethyldisilazane (HMDZ), Hexamethyldisiloxane (HMDSO), etc. Di-functional 
2 Dimethyldimethoxysilane (DMDMS), Dimethyldichlorosilane (DMDCS), Dimethyldimethoxysilane (DMMOS), etc. Tri-functional 
1 Methyltrimethoxysilane (MTMS), Methyltriethoxysilane (MTES), Methyltrichlorosilane (MTCS), Trimethoxysilane (TMMS), etc. 表 2 硅源及改性劑的成本
Table 2. Cost of silicon source and silylating agents
Name Price/yuan Specifications Silicon source Tetramethoxysilane ~276.00 /Bottle, 500 g, purity 98% Tetraethoxysilane ~56.00 /Bottle, 500 mL, AR Water glass ~1800.00 /Ton Kaolin 1000.00–2000.00 /Ton Silylating agents Trimethylchlorosilane ~49.00 /Bottle, 100 mL, purity ≥98% Methyltrimethoxysilane ~54.00 /Bottle, 100 mL Methyltriethoxysilane ~76.00 /Bottle, 100 mL, purity 98% Hexamethyldisiloxane ~67.00 /Bottle, 100 mL, purity 99% Hexamethyldisilazane ~56.00 /Bottle, 100 mL, AR, purity 98% 表 3 表面后處理法和共前驅體法對比
Table 3. Comparison between the surface posttreatment method and coprecursor method
Modification method Common drying process Modified object Advantage Disadvantage Surface posttreatment modification (surface derivation modification) Ambient pressure drying Wet gel after sol–gel process The method is simple and easy to control. The modification does not affect the original void structure. The pore size and particle size of HSA are relatively uniform The solvent consumption of the modification project is large, the internal modification is not complete, and the process is cumbersome Coprecursor modification (in situ modification) Supercritical drying Primary particles and growing aggregates in the sol–gel process With simple process flow and low cost, HSA with a large specific surface area and better hydrophobicity can be prepared The reaction process is not easy to control, the introduced hydrophobic groups are limited, and the pore size is uneven www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Adebajo M, Frost R, Kloprogge J, et al. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J Porous Mater, 2003, 10: 159 doi: 10.1023/A:1027484117065 [2] Yue X J, Zhang T, Yang D Y, et al. In situ fabrication dynamic carbon fabrics membrane with tunable wettability for selective oil-water separation. J Ind Eng Chem, 2018, 61: 188 doi: 10.1016/j.jiec.2017.12.016 [3] Jiang J X, Zhang Q H, Zhan X L, et al. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem Eng J, 2019, 358: 1539 doi: 10.1016/j.cej.2018.10.144 [4] Rao A V, Hegde N D, Hirashima H. Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J Colloid Interface Sci, 2007, 305(1): 124 doi: 10.1016/j.jcis.2006.09.025 [5] Teas C, Kalligeros S, Zanikos F, et al. Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination, 2001, 140(3): 259 doi: 10.1016/S0011-9164(01)00375-7 [6] Lin J, Zhu Y Z, Cai J Q, et al. Recovery and treatment of oil spill at sea. Fujian Energy Dev Conserv, 2001(1): 6林建, 朱躍姿, 蔡俊清, 等. 海上溢油的回收及處理. 福建能源開發與節約, 2001(1):6 [7] Zhang G P, Guo Z X, Chen H Z. Discussion on the biological purification method for the ship oil spillage. Transp Sci &Technol, 2008(3): 107 doi: 10.3963/j.issn.1671-7570.2008.03.037張國平, 郭志新, 陳厚忠. 生物處理法在船舶溢油事故中的應用探討. 交通科技, 2008(3):107 doi: 10.3963/j.issn.1671-7570.2008.03.037 [8] Zhu X B, Wang X H, Liu Y P, et al. Efficient adsorption of oil in water by hydrophobic nonwoven fabrics coated with cross-linked polydivinylbenzene fibers. J Chem Technol Biotechnol, 2018, 94(1): 128 [9] Hong J Y, Sohn E H, Park S, et al. Highly-efficient and recyclable oil absorbing performance of functionalized graphene aerogel. Chem Eng J, 2015, 269: 229 doi: 10.1016/j.cej.2015.01.066 [10] Cheng Y B, Xu P, Zeng W, et al. Highly hydrophobic and ultralight graphene aerogel as high efficiency oil absorbent material. J Environ Chem Eng, 2017, 5(2): 1957 doi: 10.1016/j.jece.2017.04.005 [11] Liu H Z, Geng B Y, Chen Y F, et al. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain Chem Eng, 2016, 5(1): 49 [12] Anderson A, Carroll M K. Hydrophobic silica aerogels: Review of synthesis, properties and applications. Aerogels handbook, 2011: 47 [13] Zhang X Y, Xu L H, Shen Y, et al. Research development of preparation and application of hydrophobic silica aerogels. Bull Chin Ceram Soc, 2017, 36(10): 3318張旋宇, 徐麗慧, 沈勇, 等. 疏水SiO2氣凝膠的制備及應用研究進展. 硅酸鹽通報, 2017, 36(10):3318 [14] Tabata M, Adachi I, Kawai H, et al. Recent progress in silica aerogel Cherenkov radiator. Phys Procedia, 2012, 37: 642 doi: 10.1016/j.phpro.2012.02.410 [15] Wernery J, Ben-Ishai A, Binder B, et al. Aerobrick—an aerogel-filled insulating brick. Energy Procedia, 2017, 134: 490 doi: 10.1016/j.egypro.2017.09.607 [16] Tang R H, Hong W, Srinivasakannan C, et al. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep Purif Technol, 2022, 281: 119950 doi: 10.1016/j.seppur.2021.119950 [17] Guo X Z, Shan J Q, Lai Z Z, et al. Facile synthesis of flexible methylsilsesquioxane aerogels with surface modifications for sound- absorbance, fast dye adsorption and oil/water separation. Molecules, 2018, 23(4): 945 doi: 10.3390/molecules23040945 [18] Amonette J E, Matyá? J. Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: A review. Microporous Mesoporous Mater, 2017, 250: 100 doi: 10.1016/j.micromeso.2017.04.055 [19] Zanini M, Lavoratti A, Lazzari L K, et al. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose, 2017, 24(2): 769 doi: 10.1007/s10570-016-1142-4 [20] Yin S, Wu H J, Ding Y F, et al. Advances in ambient-dried preparation and applications of hydrophobic SiO2 aerogels. Mater Rev, 2011, 25(3): 33殷帥, 吳會軍, 丁云飛, 等. 疏水SiO2氣凝膠的常壓制備及應用進展. 材料導報, 2011, 25(3):33 [21] Kistler S S. Coherent expanded aerogels and jellies. Nature, 1931, 127(3211): 741 [22] Li C D, Chen Z F, Yao B L, et al. Research progress of reinforced skeleton of silica aerogel. Aerosp Mater &Technol, 2019, 49(5): 1 doi: 10.12044/j.issn.1007-2330.2019.05.001李承東, 陳照峰, 姚伯龍, 等. SiO2氣凝膠骨架增強改性研究進展. 宇航材料工藝, 2019, 49(5):1 doi: 10.12044/j.issn.1007-2330.2019.05.001 [23] Wang N, Ren H B. Investigation process of silica aerogels synthesized by different types of silica recourses. Mater Rev, 2014, 28(1): 42王妮, 任洪波. 不同硅源制備二氧化硅氣凝膠的研究進展. 材料導報, 2014, 28(1):42 [24] Zhang A M. Rapid Preparation and Toughening Modification of SiO2 Aerogels [Dissertation]. Wuhan: Wuhan University of Technology, 2019張愛滿. SiO2氣凝膠的快速制備與增韌改性研究[學位論文]. 武漢: 武漢理工大學, 2019 [25] Rao A V, Kulkarni M M, Amalnerkar D P, et al. Surface chemical modification of silica aerogels using various alkyl-alkoxy/chloro silanes. Appl Surf Sci, 2003, 206(1-4): 262 doi: 10.1016/S0169-4332(02)01232-1 [26] Zuo K M, Li J, Guan Q S, et al. Preparation and application of hydrophobic aerogel. New Chem Mater, 2019, 47(4): 217左克曼, 李建, 管慶順, 等. 疏水型氣凝膠的制備及應用. 化工新型材料, 2019, 47(4):217 [27] Zhang M. Research progress in hydrophobic modification and properties of SiO2 aerogels. Mod Chem Ind, 2020, 40(1): 58張明. SiO2氣凝膠的疏水改性及性能研究進展. 現代化工, 2020, 40(1):58 [28] Anderson A M, Carroll M K, Green E C, et al. Hydrophobic silica aerogels prepared via rapid supercritical extraction. J Sol Gel Sci Technol, 2010, 53(2): 199 doi: 10.1007/s10971-009-2078-z [29] Torres R B, Vareda J P, Lamy-Mendes A, et al. Effect of different silylation agents on the properties of ambient pressure dried and supercritically dried vinyl-modified silica aerogels. J Supercrit Fluids, 2019, 147: 81 doi: 10.1016/j.supflu.2019.02.010 [30] Rao A V, Kalesh R R. Comparative studies of the physical and hydrophobic properties of TEOS based silica aerogels using different co-precursors. Sci Technol Adv Mater, 2003, 4(6): 509 doi: 10.1016/j.stam.2003.12.010 [31] Rao A V, Kalesh R. Organic surface modification of TEOS based silica aerogels synthesized by Co-precursor and derivatization methods. J Sol Gel Sci Technol, 2004, 30(3): 141 doi: 10.1023/B:JSST.0000039498.61813.9e [32] Rao A, Kulkarni M, Pajonk G, et al. Synthesis and characterization of hydrophobic silica aerogels using trimethylethoxysilane as a Co-precursor. J Sol Gel Sci Technol, 2003, 27(2): 103 doi: 10.1023/A:1023765030983 [33] Chai C, Ren H, Fan Z D. Research progress of hydrophobic modification of silica aerogels. Silicone Mater, 2020, 34(3): 62柴操, 任河, 范召東. SiO2氣凝膠疏水改性的研究進展. 有機硅材料, 2020, 34(3):62 [34] Li M, Jiang H Y, Xu D, et al. Low density and hydrophobic silica aerogels dried under ambient pressure using a new co-precursor method. J Non Cryst Solids, 2016, 452: 187 doi: 10.1016/j.jnoncrysol.2016.09.001 [35] Mahadik S A, Pedraza F, Parale V G, et al. Organically modified silica aerogel with different functional silylating agents and effect on their physico-chemical properties. J Non Cryst Solids, 2016, 453: 164 doi: 10.1016/j.jnoncrysol.2016.08.035 [36] Zhang W, Li Z, Shi L, et al. Methyltrichlorosilane modified hydrophobic silica aerogels and their kinetic and thermodynamic behaviors. J Sol Gel Sci Technol, 2019, 89(2): 448 doi: 10.1007/s10971-018-4882-9 [37] Li J P, Zhao Y Y, Ren F J, et al. Preparation of silica aerogel by different supercritical drying processes. Archit Technol, 2019, 50(8): 981李建平, 趙耀耀, 任富建, 等. 不同超臨界干燥工藝制備SiO2氣凝膠的研究. 建筑技術, 2019, 50(8):981 [38] Zhang Z D, Scherer G W. Supercritical drying of cementitious materials. Cem Concr Res, 2017, 99: 137 doi: 10.1016/j.cemconres.2017.05.005 [39] Yu H, Liang X, Wang J, et al. Preparation and characterization of hydrophobic silica aerogel sphere products by co-precursor method. Solid State Sci, 2015, 48: 155 doi: 10.1016/j.solidstatesciences.2015.08.005 [40] Lu B, Chen Q, Zeng J. Comparison of hydrophobic silica aerogels prepared by different modifiers. Mater Mech Eng, 2011, 35(6): 53盧斌, 陳琴, 曾杰. 不同改性劑制備疏水SiO2氣凝膠的對比. 機械工程材料, 2011, 35(6):53 [41] Wang J, Deng Z S, Shen J, et al. Silylation of polydiethoxysiloxane derived silica aerogels. J Non Cryst Solids, 2000, 271(1-2): 100 doi: 10.1016/S0022-3093(00)00110-1 [42] Sarawade P B, Kim J K, Kim H K, et al. High specific surface area TEOS-based aerogels with large pore volume prepared at an ambient pressure. Appl Surf Sci, 2007, 254(2): 574 doi: 10.1016/j.apsusc.2007.06.063 [43] Liu Y, Zhang Y, Li D X. Preparation of hydrophobic silica aerogels by ambitient pressure drying method. J Funct Mater, 2015, 46(5): 5132 doi: 10.3969/j.issn.1001-9731.2015.05.026劉洋, 張毅, 李東旭. 常壓干燥制備疏水性SiO2氣凝膠. 功能材料, 2015, 46(5):5132 doi: 10.3969/j.issn.1001-9731.2015.05.026 [44] He S, Li Z, Shi X J, et al. Rapid synthesis of sodium silicate based hydrophobic silica aerogel granules with large surface area. Adv Powder Technol, 2015, 26(2): 537 doi: 10.1016/j.apt.2015.01.002 [45] Hu W B, Li M M, Chen W, et al. Preparation of hydrophobic silica aerogel with Kaolin dried at ambient pressure. Colloids Surf A Physicochem Eng Aspects, 2016, 501: 83 doi: 10.1016/j.colsurfa.2016.04.059 [46] Lu B, Sun J Y, Wei Q Q, et al. Effect of acids on properties of silica aerogels prepared from silica Sol at ambient pressure. J Chin Ceram Soc, 2013, 41(2): 153盧斌, 孫俊艷, 魏琪青, 等. 酸種類對以硅溶膠為原料、常壓制備的SiO2氣凝膠性能的影響. 硅酸鹽學報, 2013, 41(2):153 [47] Rao A P, Rao A V, Pajonk G M. Hydrophobic and physical properties of the two step processed ambient pressure dried silica aerogels with various exchanging solvents. J Sol Gel Sci Technol, 2005, 36(3): 285 doi: 10.1007/s10971-005-4662-1 [48] Bi H J, Huang D M, He S, et al. Effect of modifier on the characteristics of silica aerogels. J Mater Sci Eng, 2014, 32(2): 178 doi: 10.14136/j.cnki.issn1673-2812.2014.02.005畢海江, 黃冬梅, 何松, 等. 改性劑對二氧化硅氣凝膠性能的影響. 材料科學與工程學報, 2014, 32(2):178 doi: 10.14136/j.cnki.issn1673-2812.2014.02.005 [49] Schwertfeger F, Frank D, Schmidt M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J Non Cryst Solids, 1998, 225: 24 doi: 10.1016/S0022-3093(98)00102-1 [50] Shi F, Wang L J, Liu J X. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater Lett, 2006, 60(29-30): 3718 doi: 10.1016/j.matlet.2006.03.095 [51] Lee C M, Kim G S, Hyun S. Synthesis of silica aerogels from waterglass via new modified ambient drying. J Mater Sci, 2002, 37: 2237 doi: 10.1023/A:1015309014546 [52] Bhagat S D, Kim Y H, Suh K H, et al. Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater, 2008, 112(1-3): 504 doi: 10.1016/j.micromeso.2007.10.030 [53] Shewale P M, Rao A V, Rao A P. Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels. Appl Surf Sci, 2008, 254(21): 6902 doi: 10.1016/j.apsusc.2008.04.109 [54] Mahadik D B, Rao A V, Rao A P, et al. Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J Colloid Interface Sci, 2011, 356(1): 298 doi: 10.1016/j.jcis.2010.12.088 [55] Rao A P, Rao A V. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J Mater Sci, 2010, 45(1): 51 doi: 10.1007/s10853-009-3888-7 [56] Wei T Y, Chang T F, Lu S Y, et al. Preparation of monolithic silica aerogel of low thermal conductivity by ambient pressure drying. J Am Ceram Soc, 2007, 90(7): 2003 doi: 10.1111/j.1551-2916.2007.01671.x [57] Gan L H, Zhang Y X, Chen L W, et al. Application of drying control chemical additives in preparation for silica aerogels. J Tongji Univ, 2003, 31(9): 1131 doi: 10.3321/j.issn:0253-374X.2003.09.026甘禮華, 張宇星, 陳龍武, 等. 干燥控制化學添加劑在制備硅氣凝膠中的應用. 同濟大學學報(自然科學版), 2003, 31(9):1131 doi: 10.3321/j.issn:0253-374X.2003.09.026 [58] Liu M L, Yang D A, Qu Y F. Preparation of super hydrophobic silica aerogel and study on its fractal structure. J Non Cryst Solids, 2008, 354(45-46): 4927 doi: 10.1016/j.jnoncrysol.2008.06.023 [59] Lenza R F S, Nunes E H M, Vasconcelos D C L, et al. Preparation of Sol-gel silica samples modified with drying control chemical additives. J Non Cryst Solids, 2015, 423-424: 35 doi: 10.1016/j.jnoncrysol.2015.05.010 [60] Omranpour H, Motahari S. Effects of processing conditions on silica aerogel during aging: Role of solvent, time and temperature. J Non Cryst Solids, 2013, 379: 7 doi: 10.1016/j.jnoncrysol.2013.07.025 [61] Omranpour H, Dourbash A, Motahari S. Mechanical properties improvement of silica aerogel through aging: Role of solvent type, time and temperature. AIP Conf Proc, 2014, 1593(1): 298 [62] Rao A V, Sakhare H M, Tamhankar A K, et al. Influence of N, N-dimethylformamide additive on the physical properties of citric acid catalyzed TEOS silica aerogels. Mater Chem Phys, 1999, 60(3): 268 doi: 10.1016/S0254-0584(99)00089-9 [63] Schwertfeger F, Glaubitt W, Schubert U. Hydrophobic aerogels from Si(OMe)4/MeSi(OMe)3 mixtures. J Non Cryst Solids, 1992, 145: 85 doi: 10.1016/S0022-3093(05)80435-1 [64] Liu J L, Guo W B, Bai X, et al. Research progress on modified silica aerogels. Guangzhou Chem Ind, 2019, 47(6): 16 doi: 10.3969/j.issn.1001-9677.2019.06.012劉金玲, 郭慰彬, 白欣, 等. 改性二氧化硅氣凝膠研究進展. 廣州化工, 2019, 47(6):16 doi: 10.3969/j.issn.1001-9677.2019.06.012 [65] Wu G Y, Yu Y X, Cheng X, et al. Structure and properties of monolithic silica aerogels by methyltrimethoxysilane. J Chin Ceram Soc, 2009, 37(7): 1206 doi: 10.3321/j.issn:0454-5648.2009.07.026吳國友, 余煜璽, 程璇, 等. 甲基三甲氧基硅烷對塊狀SiO2氣凝膠性能和結構的影響. 硅酸鹽學報, 2009, 37(7):1206 doi: 10.3321/j.issn:0454-5648.2009.07.026 [66] Parale V G, Lee K Y, Jung H N R, et al. Facile synthesis of hydrophobic, thermally stable, and insulative organically modified silica aerogels using co-precursor method. Ceram Int, 2018, 44(4): 3966 doi: 10.1016/j.ceramint.2017.11.189 [67] Ren H B, Zhu J Y, Bi Y T, et al. One-step fabrication of transparent hydrophobic silica aerogels via in situ surface modification in drying process. J Sol Gel Sci Technol, 2016, 80(3): 635 doi: 10.1007/s10971-016-4146-5 [68] Chen Y Z, Ou Z W, Liu Z H. Preparation and properties of self-hydrophobic silica aerogel based on methltrimethoxysilane/water glass. J Chin Ceram Soc, 2018, 46(4): 511陳宇卓, 歐忠文, 劉朝輝. 甲基三甲氧基硅烷改性水玻璃基自疏水SiO2氣凝膠的制備. 硅酸鹽學報, 2018, 46(4):511 [69] Bhagat S D, Oh C S, Kim Y H, et al. Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying. Microporous Mesoporous Mater, 2007, 100(1-3): 350 doi: 10.1016/j.micromeso.2006.10.026 [70] Li G A, Zhu T L, Ye L Y, et al. Hydrophobic silica aerogel prepared In-situ by ambient pressure drying and its thermal stability. Acta Phys Chimica Sin, 2009, 25(9): 1811 doi: 10.3866/PKU.WHXB20090902李貴安, 朱庭良, 葉錄元, 等. 原位法常壓干燥制備疏水SiO2氣凝膠及其熱穩定性. 物理化學學報, 2009, 25(9):1811 doi: 10.3866/PKU.WHXB20090902 [71] Cai L, Shan G R. Elastic silica aerogel using methyltrimethoxysilane precusor via ambient pressure drying. J Porous Mater, 2015, 22(6): 1455 doi: 10.1007/s10934-015-0026-6 [72] Zhang M, Shi T J, Wen T L, et al. Preparation and performance characterization of SiO2 aerogel by multi-hydrophobic modification. Chem Propellants &Polym Mater, 2013, 11(1): 72張明, 史鐵鈞, 文聽雷, 等. 多次疏水改性SiO2氣凝膠的制備及其性能表征. 化學推進劑與高分子材料, 2013, 11(1):72 [73] Bhagat S D, Kim Y H, Moon M J, et al. A cost-effective and fast synthesis of nanoporous SiO2 aerogel powders using water-glass via ambient pressure drying route. Solid State Sci, 2007, 9(7): 628 doi: 10.1016/j.solidstatesciences.2007.04.020 [74] Matias T, Varino C, Sousa H C, et al. Novel flexible, hybrid aerogels with vinyl- and methyltrimethoxysilane in the underlying silica structure. J Mater Sci, 2016, 51(14): 6781 doi: 10.1007/s10853-016-9965-9 [75] Fátima Júlio M, Ilharco L M. Ambient pressure hybrid silica monoliths with hexamethyldisilazane: From vitreous hydrophilic xerogels to superhydrophobic aerogels. ACS Omega, 2017, 2(8): 5060 doi: 10.1021/acsomega.7b00893 [76] Wu C H, Li K F, Li X H, et al. Research progress on preparation of silica aerogels at ambient pressure drying. Chem Ind Eng Prog, 2022, 41(2): 837武晨浩, 李昆鋒, 李肖華, 等. 二氧化硅氣凝膠常壓干燥工藝的研究進展. 化工進展, 2022, 41(2):837 [77] ?losarczyk A, Bare?kowski M, Niemier S, et al. Synthesis and characterisation of silica aerogel/carbon microfibers nanocomposites dried in supercritical and ambient pressure conditions. J Sol Gel Sci Technol, 2015, 76(1): 227 doi: 10.1007/s10971-015-3798-x [78] Shajesh P, Smitha S, Aravind P R, et al. Synthesis, structure and properties of cross-linked R(SiO1.5)/SiO2 (R = 3-glycidoxypropyl) porous organic inorganic hybrid networks dried at ambient pressure. J Colloid Interface Sci, 2009, 336(2): 691 doi: 10.1016/j.jcis.2009.04.023 [79] Yun S, Luo H J, Gao Y F. Ambient-pressure drying synthesis of large resorcinol-formaldehyde-reinforced silica aerogels with enhanced mechanical strength and superhydrophobicity. J Mater Chem A, 2014, 2(35): 14542 doi: 10.1039/C4TA02195A [80] Zhang Z H, Shen J, Ni X Y, et al. Preparation and characterization of hydrophobic silica aerogels doped with fibers. Rare Met Mater Eng, 2008(S2): 16張志華, 沈軍, 倪星元, 等. 纖維摻雜疏水SiO2氣凝膠的制備與表征(英文). 稀有金屬材料與工程, 2008(S2):16 [81] Linhares T, Amorim M T P, Dur?es L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J Mater Chem A, 2019, 7(40): 22768 doi: 10.1039/C9TA04811A [82] Zhou T, Cheng X D, Pan Y L, et al. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel composites based on co-precursor method by freeze drying. Appl Surf Sci, 2018, 437: 321 doi: 10.1016/j.apsusc.2017.12.146 [83] Lin J M, Li G Z, Liu W, et al. A review of recent progress on the silica aerogel monoliths: Synthesis, reinforcement, and applications. J Mater Sci, 2021, 56(18): 10812 doi: 10.1007/s10853-021-05997-w [84] Patil S P, Shendye P, Markert B. Molecular dynamics simulations of silica aerogel nanocomposites reinforced by glass fibers, graphene sheets and carbon nanotubes: A comparison study on mechanical properties. Compos B Eng, 2020, 190: 107884 doi: 10.1016/j.compositesb.2020.107884 [85] ?losarczyk A. Carbon fiber-silica aerogel composite with enhanced structural and mechanical properties based on water glass and ambient pressure drying. Nanomaterials (Basel), 2021, 11(2): 258 doi: 10.3390/nano11020258 [86] Li C C, Cheng X D, Li Z, et al. Mechanical, thermal and flammability properties of glass fiber film/silica aerogel composites. J Non Cryst Solids, 2017, 457: 52 doi: 10.1016/j.jnoncrysol.2016.11.017 [87] He X, Zhou Q X. Research progress of new petroleum adsorbents based on chitosan aerogels. J Zhejiang Univ (Eng Sci), 2021, 55(7): 1368 doi: 10.3785/j.issn.1008-973X.2021.07.016何璇, 周啟星. 基于殼聚糖氣凝膠的新型石油吸附劑研究進展. 浙江大學學報(工學版), 2021, 55(7):1368 doi: 10.3785/j.issn.1008-973X.2021.07.016 [88] Ma Q, Liu Y F, Dong Z, et al. Hydrophobic and nanoporous chitosan-silica composite aerogels for oil absorption. J Appl Polym Sci, 2015, 132(15): 41770 [89] Liu H L, Chu P, Li H Y, et al. Preparation and properties of silica aerogels reinforced using polyurethanes via ambient pressure drying. J Synth Cryst, 2015, 44(12): 3532 doi: 10.3969/j.issn.1000-985X.2015.12.030劉洪麗, 褚鵬, 李洪彥, 等. 聚氨酯增強二氧化硅氣凝膠常壓干燥制備及其性能. 人工晶體學報, 2015, 44(12):3532 doi: 10.3969/j.issn.1000-985X.2015.12.030 [90] Mahadik D B, Jung H N R, Han W, et al. Flexible, elastic, and superhydrophobic silica-polymer composite aerogels by high internal phase emulsion process. Compos Sci Technol, 2017, 147: 45 doi: 10.1016/j.compscitech.2017.04.036 [91] Gurav J L, Rao A V, Nadargi D Y, et al. Ambient pressure dried TEOS-based silica aerogels: Good absorbents of organic liquids. J Mater Sci, 2010, 45(2): 503 doi: 10.1007/s10853-009-3968-8 [92] He S, Huang D M, Bi H J, et al. Synthesis and characterization of silica aerogels dried under ambient pressure bed on water glass. J Non Cryst Solids, 2015, 410: 58 doi: 10.1016/j.jnoncrysol.2014.12.011 [93] Reynolds J G, Coronado P R, Hrubesh L W. Hydrophobic aerogels for oil-spill clean up - synthesis and characterization. J Non Cryst Solids, 2001, 292(1-3): 127 doi: 10.1016/S0022-3093(01)00882-1 [94] Wei Y, Wang J, Zhang Y L, et al. Autocatalytic synthesis of molecular-bridged silica aerogels with excellent absorption and super elasticity. RSC Adv, 2015, 5(111): 91407 doi: 10.1039/C5RA19776G [95] Wu Z Y, Li C, Liang H W, et al. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angewandte Chemie Int Ed, 2013, 52(10): 2925 doi: 10.1002/anie.201209676 [96] Liu Y F. Synthesis, Modification and Properties of Gelatin–Silica Composite Aerogels [Dissertation]. Tianjin: Tianjin University, 2016劉燕飛. 明膠–二氧化硅復合氣凝膠的制備、改性及其性能研究[學位論文]. 天津: 天津大學, 2016 [97] Renjith P K, Sarathchandran C, Sivanandan Achary V, et al. Micro-cellular polymer foam supported silica aerogel: Eco-friendly tool for petroleum oil spill cleanup. J Hazard Mater, 2021, 415: 125548 doi: 10.1016/j.jhazmat.2021.125548 [98] Liu G Q, Yang R, Li M. Porous structure and adsorption behaviors of silica aerogels. J Chin Ceram Soc, 2009, 37(4): 516 doi: 10.3321/j.issn:0454-5648.2009.04.006劉國強, 楊儒, 李敏. 二氧化硅氣凝膠的孔結構及液相吸附行為. 硅酸鹽學報, 2009, 37(4):516 doi: 10.3321/j.issn:0454-5648.2009.04.006 [99] Parale V G, Mahadik D B, Kavale M S, et al. Potential application of silica aerogel granules for cleanup of accidental spillage of various organic liquids. Soft Nanosci Lett, 2011, 1(4): 97 doi: 10.4236/snl.2011.14017 [100] ?ok S S, Ko? F, Gi?zli? N. Lightweight and highly hydrophobic silica aerogels dried in ambient pressure for an efficient oil/organic solvent adsorption. J Hazard Mater, 2021, 408: 124858 doi: 10.1016/j.jhazmat.2020.124858 [101] Liu Y T, Sun J W, Yuan J G, et al. A type of thiophene-bridged silica aerogel with a high adsorption capacity for organic solvents and oil pollutants. Inorg Chem Front, 2018, 5(8): 1894 doi: 10.1039/C8QI00360B [102] Quevedo J A, Patel G, Pfeffer R. Removal of oil from water by inverse fluidization of aerogels. Ind Eng Chem Res, 2008, 48(1): 191 [103] Wang D, Silbaugh T, Pfeffer R, et al. Removal of emulsified oil from water by inverse fluidization of hydrophobic aerogels. Powder Technol, 2010, 203(2): 298 doi: 10.1016/j.powtec.2010.05.021 [104] Mazrouei-Sebdani Z, Salimian S, Khoddami A, et al. Sodium silicate based aerogel for absorbing oil from water: The impact of surface energy on the oil/water separation. Mater Res Express, 2019, 6(8): 85059 doi: 10.1088/2053-1591/ab1eed [105] Wang J T, Wang H F. Facile synthesis of flexible mesoporous aerogel with superhydrophobicity for efficient removal of layered and emulsified oil from water. J Colloid Interface Sci, 2018, 530: 372 doi: 10.1016/j.jcis.2018.07.002 [106] Zhang Y, Xiang L, Shen Q Q, et al. Rapid synthesis of dual-mesoporous silica aerogel with excellent adsorption capacity and ultra-low thermal conductivity. J Non Cryst Solids, 2021, 555: 120547 doi: 10.1016/j.jnoncrysol.2020.120547 [107] Mahani A A, Motahari S, Mohebbi A. Sol-gel derived flexible silica aerogel as selective adsorbent for water decontamination from crude oil. Mar Pollut Bull, 2018, 129(2): 438 doi: 10.1016/j.marpolbul.2017.10.012 [108] Wang D, McLaughlin E, Pfeffer R, et al. Adsorption of oils from pure liquid and oil-water emulsion on hydrophobic silica aerogels. Sep Purif Technol, 2012, 99: 28 doi: 10.1016/j.seppur.2012.08.001 [109] Yun S, Luo H J, Gao Y F. Superhydrophobic silica aerogel microspheres from methyltrimethoxysilane: Rapid synthesis via ambient pressure drying and excellent absorption properties. RSC Adv, 2014, 4(9): 4535 doi: 10.1039/C3RA46911E [110] He S. Preparation of Silica Aerogel and Its Composites for Adsorption Application [Dissertation]. Hefei: University of Science and Technology of China, 2016何松. 二氧化硅氣凝膠及其復合材料制備與吸附應用研究[學位論文]. 合肥: 中國科學技術大學, 2016 [111] Yun L, Zhao J, Kang X L, et al. Preparation and properties of monolithic and hydrophobic gelatin-silica composite aerogels for oil absorption. J Sol Gel Sci Technol, 2017, 83(1): 197 doi: 10.1007/s10971-017-4378-z [112] Zhou Y H, Chen X Y, Zuo C, et al. Performances of waste paper cellulose/SiO2 composite aerogel. CIESC J, 2019, 70(3): 1120周鈺寒, 陳曉玉, 左成, 等. 廢紙纖維素/SiO2復合氣凝膠的性能. 化工學報, 2019, 70(3):1120 [113] Shi M J, Tang C G, Yang X D, et al. Superhydrophobic silica aerogels reinforced with polyacrylonitrile fibers for adsorbing oil from water and oil mixtures. RSC Adv, 2017, 7(7): 4039 doi: 10.1039/C6RA26831E -




 下載:
下載: