-
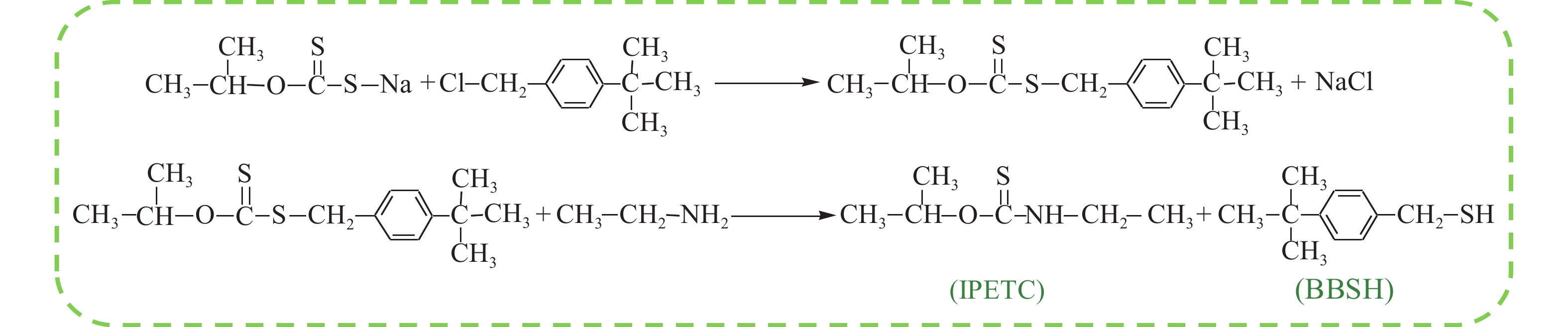
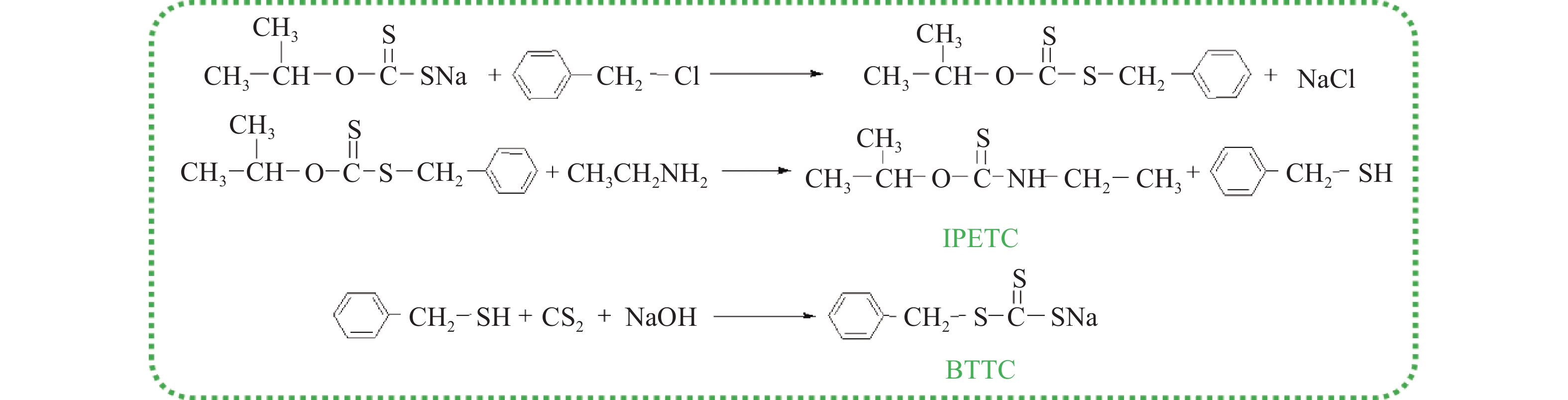
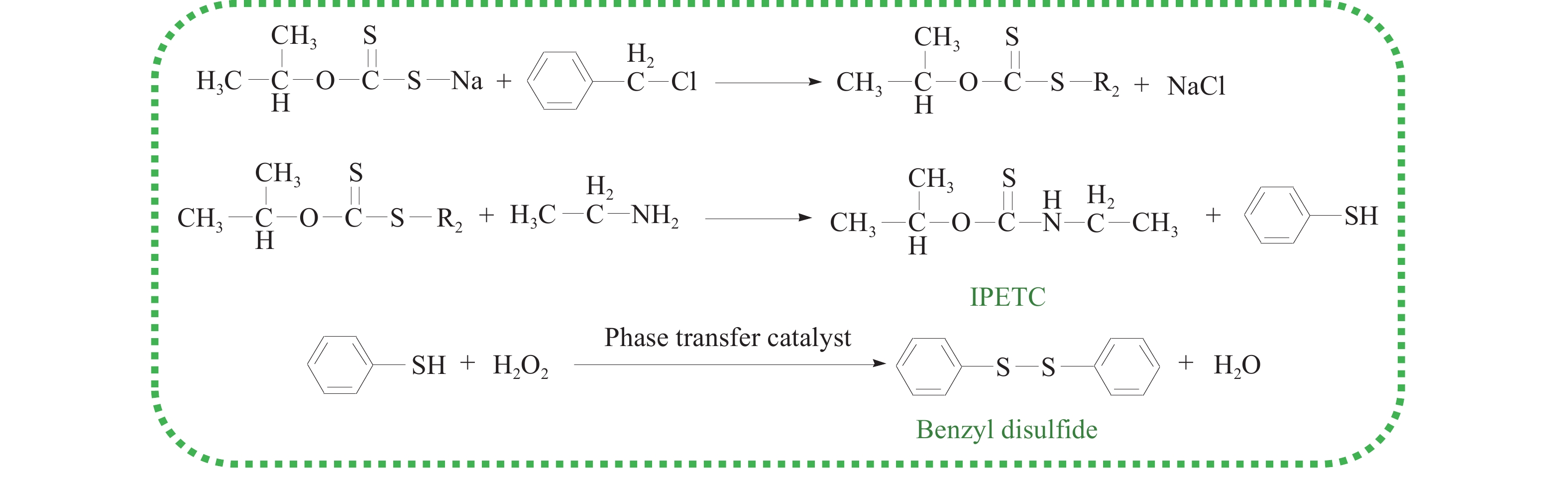
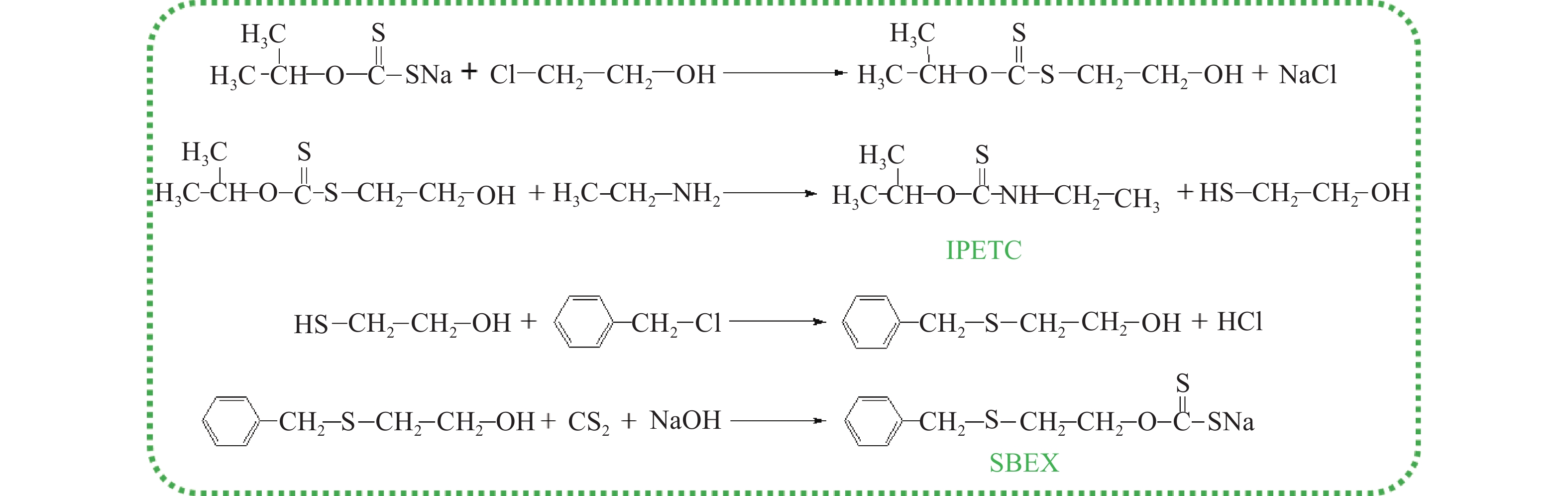
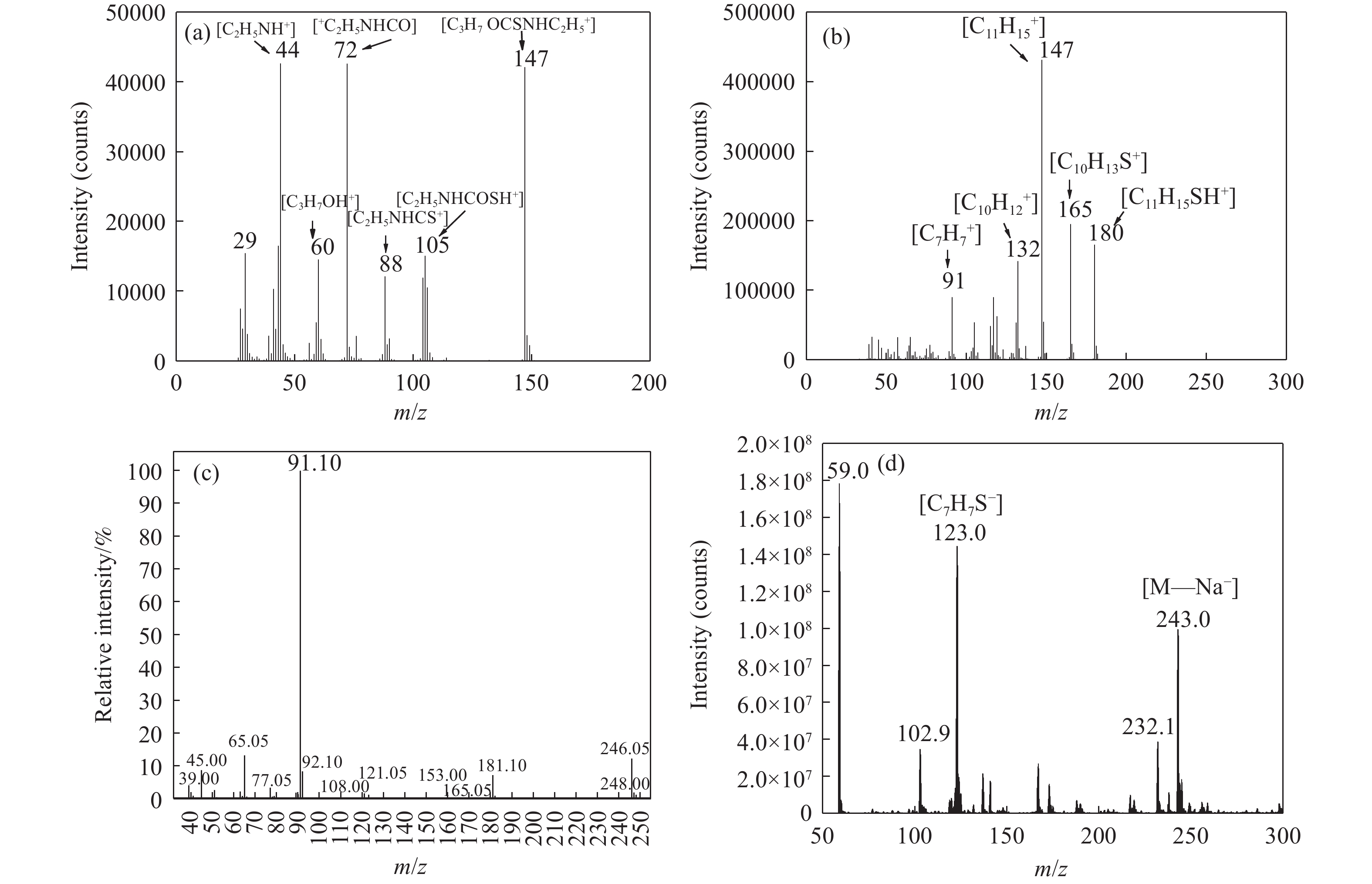
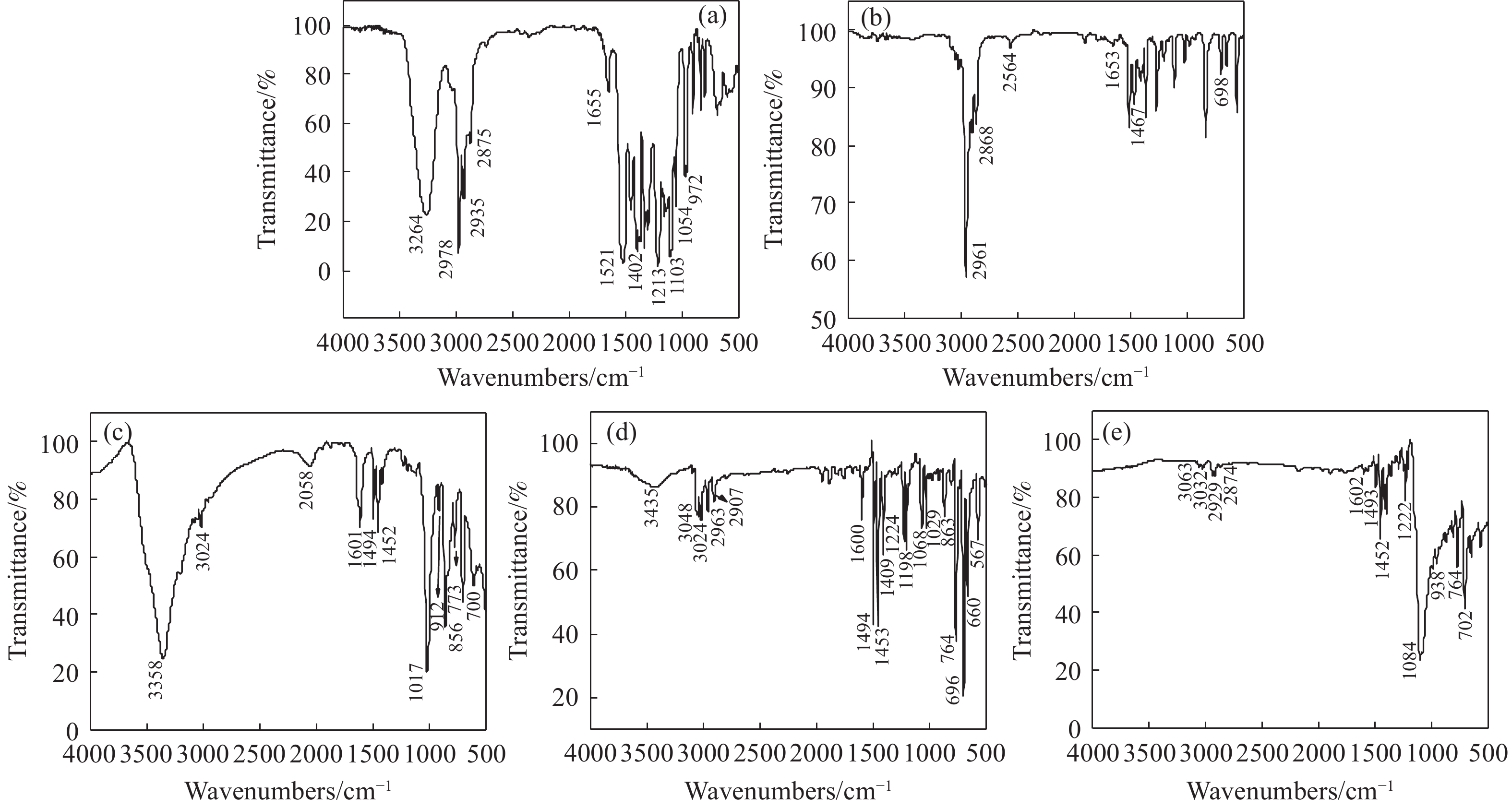
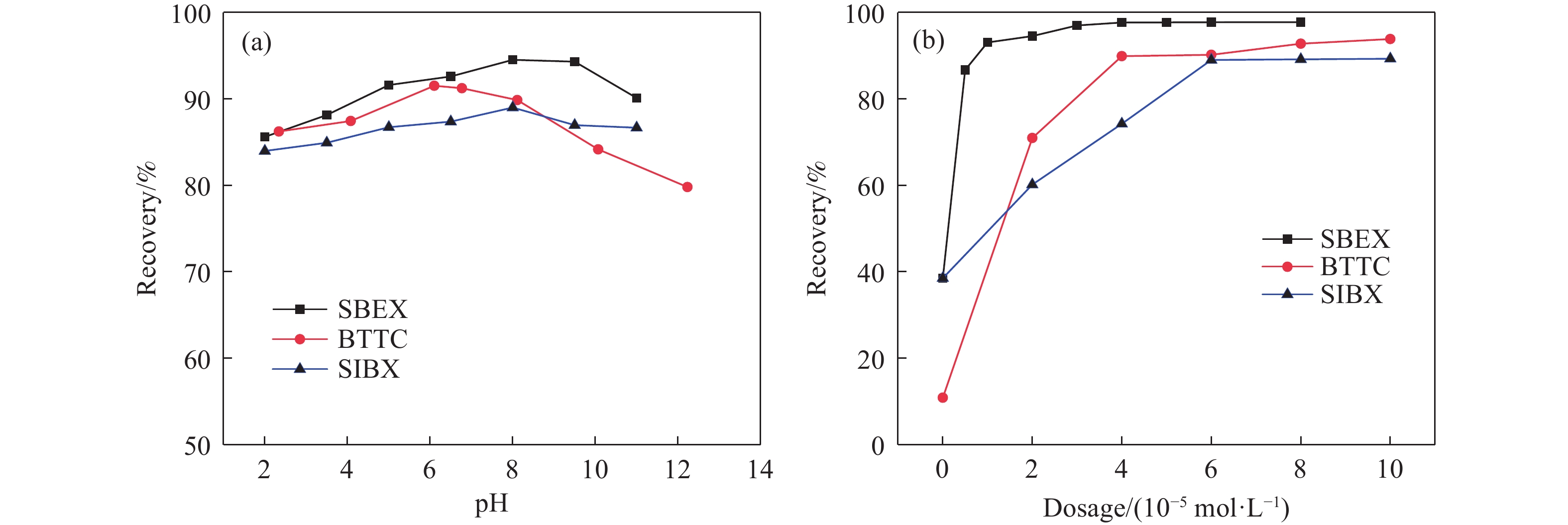
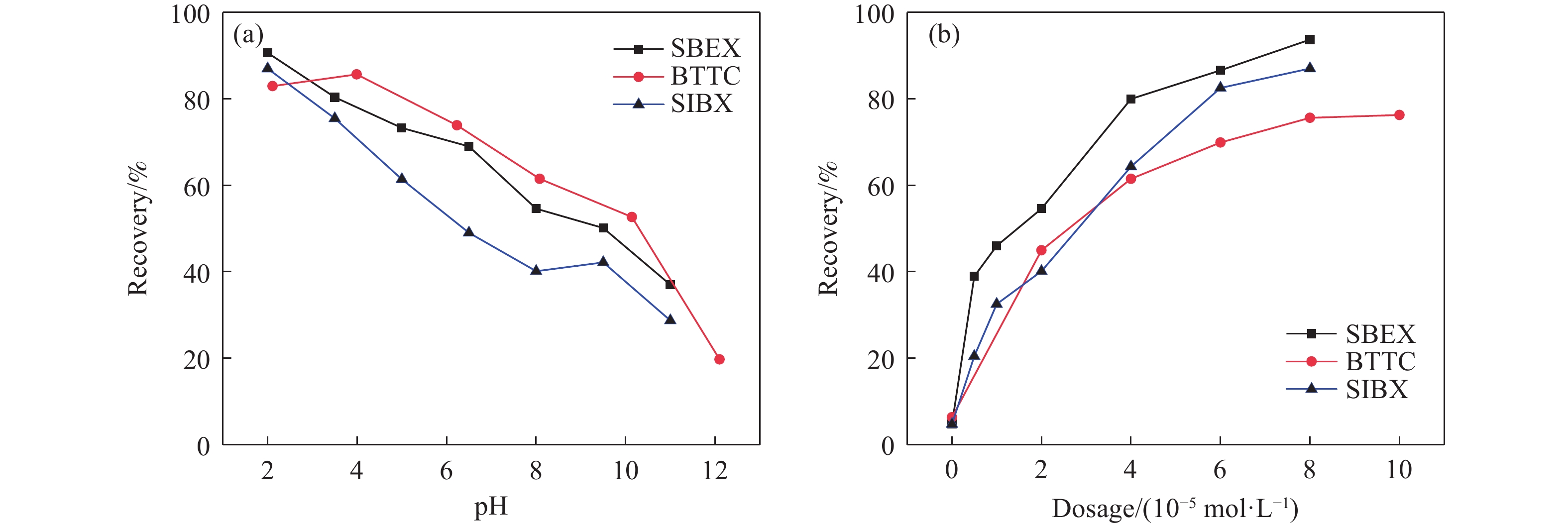
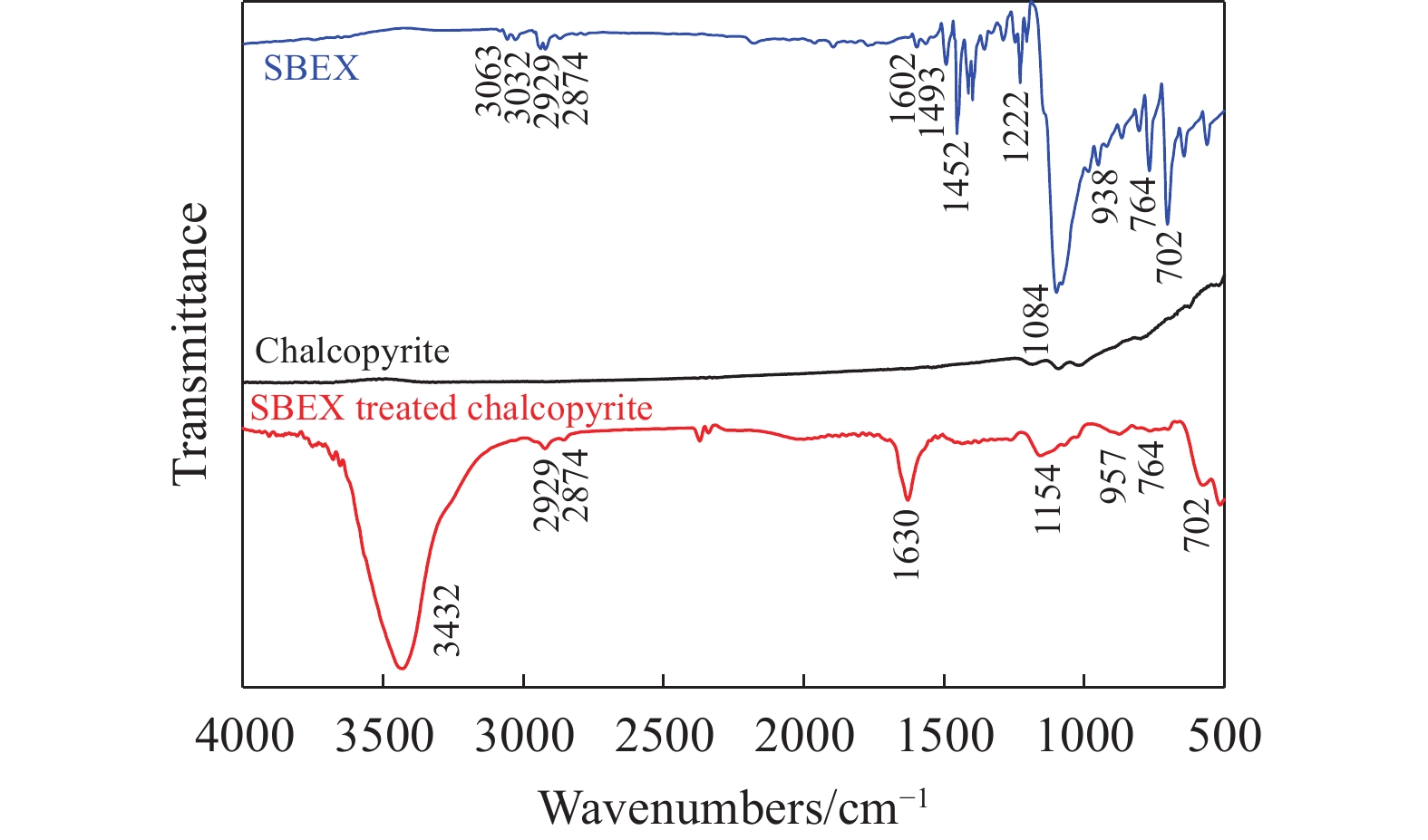
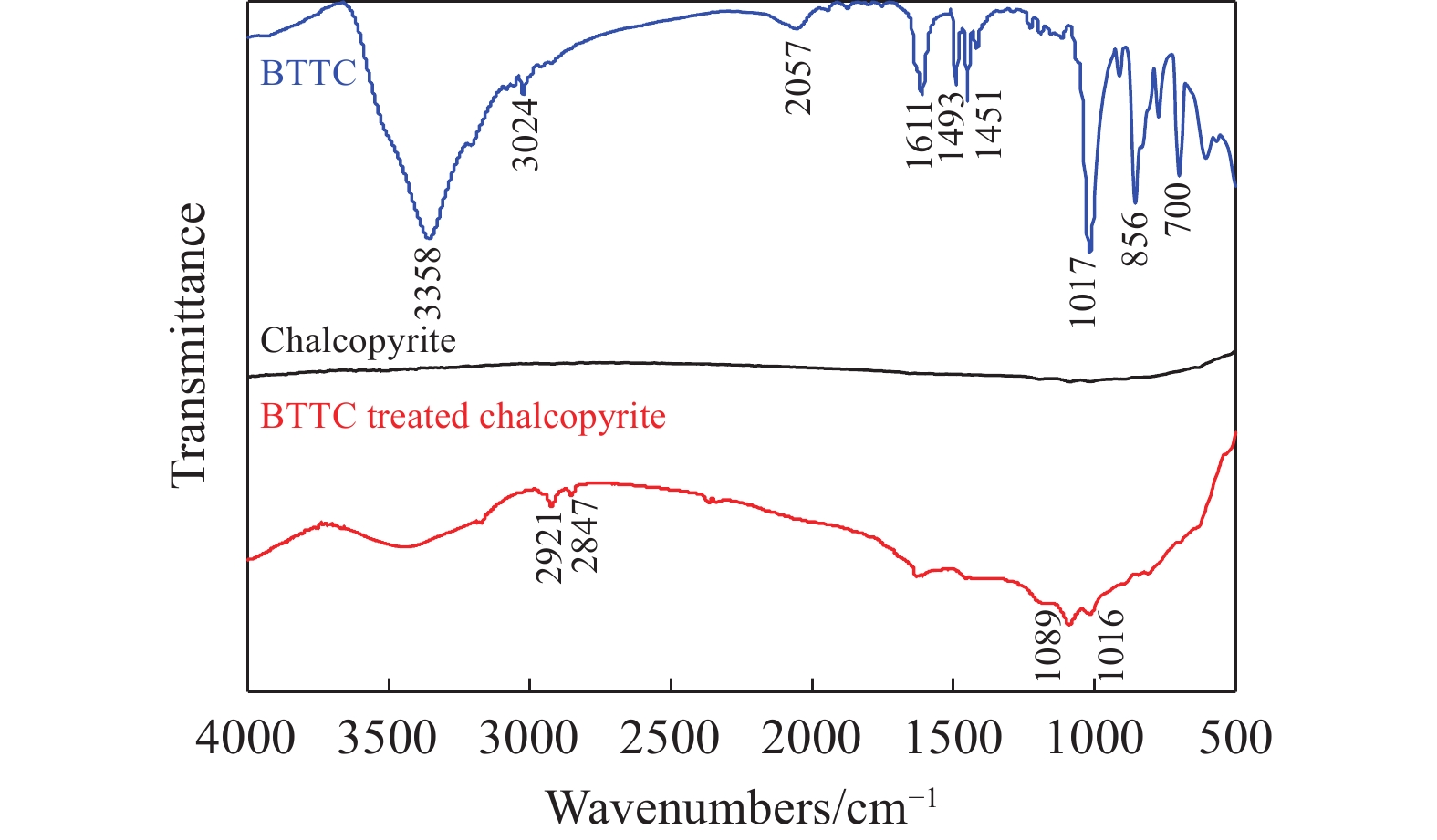
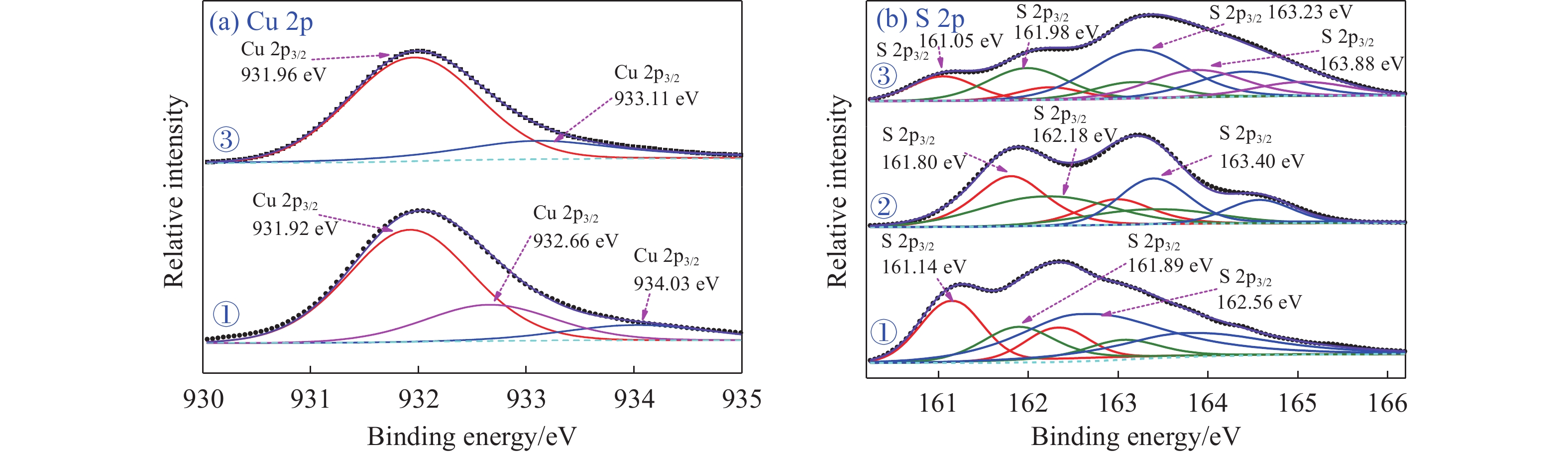
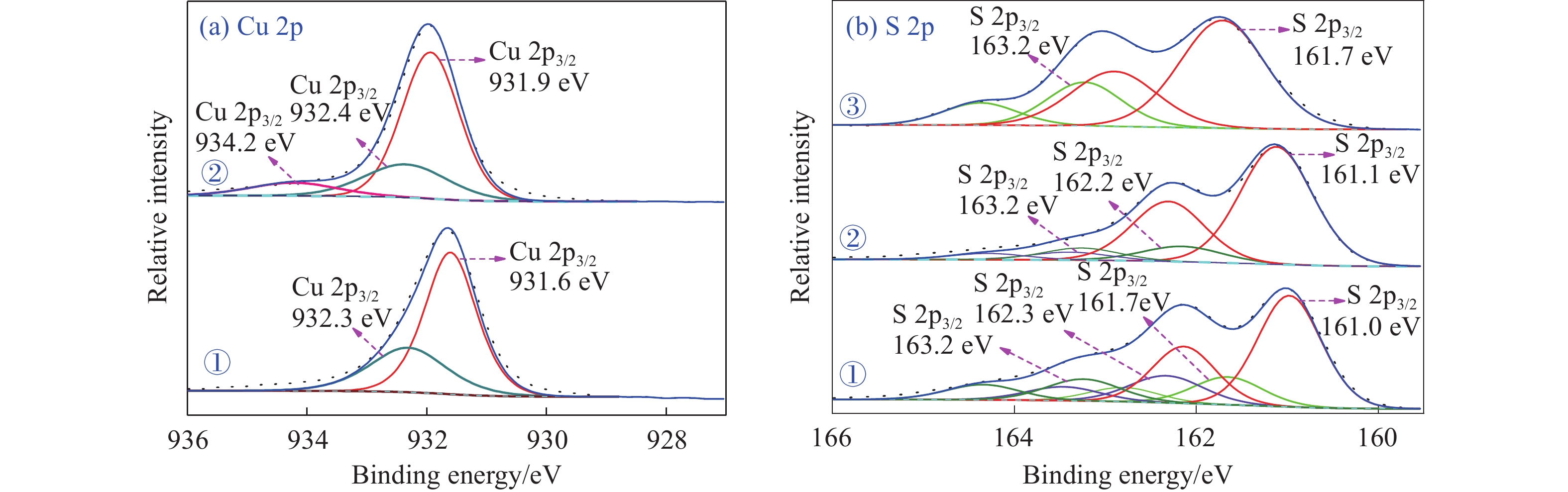
摘要: 為解決硫氨酯捕收劑制備過程中副產品處理困難、存在污染等問題,設計了四種新工藝制備乙硫氨酯(IPETC),分別聯產對叔丁基芐基硫醇(BBSH)、芐基三硫代碳酸鹽(BTTC)、芐硫基乙基黃藥(SBEX)、二芐基二硫醚。在優化的合成工藝條件下,合成IPETC聯產BBSH,得到含IPETC和BBSH的復合捕收劑,其中IPETC的質量分數為51%,BBSH的質量分數為41%,IPETC和BBSH的收率達到95%;合成IPETC聯產BTTC,IPETC和BTTC的收率分別達到94%和95%,純度分別為91%和82%;合成IPETC聯產SBEX,IPETC的收率和純度分別達到89%和95%,SBEX的收率和純度分別為93%和91%;合成IPETC聯產二芐基二硫醚,IPETC的收率和純度分別達到93%和92%,二芐基二硫醚的收率和純度分別達到95%和94%。考察了制備的復合捕收劑(IPETC與BBSH)對銅鉬礦的浮選性能,結果表明,復合捕收劑對銅鉬礦表現出良好的捕收性能。聯產的新型捕收劑SBEX、BTTC對黃銅礦的捕收力略強于異丁基黃藥,對黃鐵礦具有較好的選擇性,可替代異丁基黃藥浮選硫化銅礦。紅外光譜和X射線光電子能譜分析結果表明,SBEX、BTTC與黃銅礦作用時,捕收劑分子中的C=S和C—S與礦物表面的金屬Cu作用,生成捕收劑與銅的表面絡合物吸附在黃銅礦的表面。Abstract: To address the problems of byproduct treatment and pollution in thionocarbamate preparation, four novel processes for preparing O-isopropyl-N-ethyl thionocarbamate (IPETC) were designed, which can coproduce 4-(tert-butyl)benzyl mercaptan (BBSH), sodium benzyl trithiocarbonate (BTTC), sodium O-benzylthioethyl xanthate (SBEX), and benzyl disulfide, respectively. All the products were confirmed via FTIR and mass spectrometry. The composite collector (IPETC and BBSH mass contents were 51% and 41%, respectively) was synthesized via one-pot reaction of sodium isopropyl xanthate, 4-tert-butylbenzylchloride, and ethylamine using tert-butyl alcohol as solvent. The yield of IPETC and BBSH was 95% in the process of coproducing IPETC and BBSH. Specifically, BTTC and IPETC were synthesized via a reaction of sodium isopropyl xanthate, benzyl chloride, ethylamine, carbon disulfide, and sodium hydroxide. The IPETC and BTTC yields were 94% and 95% with a purity of 91% and 82% in the process of coproducing IPETC and BTTC, respectively. Meanwhile, SBEX and IPETC were synthesized via reaction of sodium isopropyl xanthate, 2-chloroethanol, ethylamine, benzyl chloride, carbon disulfide, and sodium hydroxide. The IPETC and SBEX yields were 89% and 93% with a purity of 95% and 91% in the process of coproducing IPETC and SBEX, respectively. Benzyl disulfide and IPETC were synthesized via a reaction of sodium isopropyl xanthate, benzyl chloride, ethylamine, and hydrogen peroxide. The IPETC and benzyl disulfide yields were 93% and 95% with a purity of 92% and 94% in the processof coproducing IPETC and benzyl disulfide, respectively. The flotation response of copper-molybdenum ore independent with IPETC and BBSH collectors and with their mixture was assessed. The flotation results indicate that the composite collector displays a superior collecting capability for copper sulfide ore. Further, the combination of IPETC and BBSH could give rise to a synergistic effect, significantly enhancing the overall flotation performance. The flotation performance of SBEX and BTTC on chalcopyrite and pyrite was also investigated. The flotation results indicate that SBEX and BTTC exhibited better collecting performance than sodium isobutyl xanthate (SIBX), which can replace SIBX for the flotation separation of copper sulfide. FTIR spectra and X-ray photoelectron spectroscopy analyses were conducted. The results indicate that when all three sulfur atoms in BTTC bond to the mineral surface, the hydrophobicity increases when compared to xanthates, wherein oxygen does not bond to the surface. Further, the thioether structure can increase the hydrophobicity of SBEX on the chalcopyrite surface, and SBEX features a higher collecting recovery toward chalcopyrite than SIBX. The results indicate that BTTC and SBEX might bond with copper atoms on the chalcopyrite surface through their sulfur atoms to form BTTC-Cu and SBEX-Cu surface complexes.
-
Key words:
- flotation /
- thionocarbamates /
- trithiocarbonates /
- mercaptans /
- disulfides /
- collector
-
表 1 捕收劑對內蒙古某銅鉬礦的一次粗選實驗結果
Table 1. Flotation conditions and results of copper-molybdenum ore from Inner Mongolia Copper-Molybdenum Mine
Entry Reagents and their dosages/(g·t?1) Products Yield/% Grade/% Recovery/% Cu Mo Cu Mo 1 Composite collector, 24
Pine oil, 24Concentrates 5.64 3.99 0.44 76.08 70.90 Tailings 94.36 0.075 0.011 23.92 29.10 Feed 100.00 0.296 0.035 100.00 100.00 2 IPETC 24
Pine oil, 24Concentrates 6.07 3.69 0.29 74.91 60.70 Tailings 93.93 0.08 0.012 25.09 39.30 Feed 100.00 0.299 0.029 100.00 100.00 3 BBSH, 24
Pine oil, 24Concentrates 6.98 3.11 0.31 72.11 69.80 Tailings 93.02 0.09 0.010 27.89 30.20 Feed 100.00 0.301 0.031 100.00 100.00 www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Bu Y J, Hu Y H, Sun W, et al. Fundamental flotation behaviors of chalcopyrite and galena using O-isopropyl-N-ethyl thionocarbamate as a collector. Minerals, 2018, 8(3): 115 doi: 10.3390/min8030115 [2] Fischback B C, Harris G H. Process for the Manufacture of Dialkyl Thionocarbamates: US Patent, 2691635. 1954-10-12 [3] Hamm P C. Destroying Vegetation with Haloalkenyl Disubstituted Thionocarbamates: US Patent, 3224864. 1965-12-21 [4] Bishop M D, Gray L A. Catalytic Synthesis of Thionocarbamates from Xanthates and Amines: US Patent, 5041599. 1991-08-20 [5] Dai H Y, Wang M J. Process of Preparing Ethyl Ammonia Sulfate: China Patent, 96110154. 1998-01-14戴洪義, 王美君. 乙硫氨酯制備的新工藝: 中國專利, 96110154. 1998-01-14 [6] Lewellyn M E. Process for the Preparation of N-Allyl-O-Alkyl Thionocarbamates: US Patent, 4482500. 1984-11-13 [7] Luan H L, Yao W, Wu R C. Prepn of Thioamino-Formates Compounds: China Patent, 96106463. 1997-07-09欒和林, 姚文, 武榮成. 一種硫代胺基甲酸酯類化合物的制備方法: 中國專利, 96106463. 1997-07-09 [8] Xu Q H. Experimental study on extraction process of recovery of thioglycolic acid from the sulfur ammonia ester solution. Shandong Chem Ind, 2015, 44(12): 10徐慶華. 從硫氨酯尾液中回收巰基乙酸萃取工藝的實驗研究. 山東化工, 2015, 44(12):10 [9] Wan S H, Liu G Y, Wang D T, et al. Compounding process of thioglycolic acid using as depressant against copper sulfides. Copp Eng, 2006(4): 68萬盛輝, 劉廣義, 王德庭, 等. 銅抑制劑巰基乙酸的合成工藝. 銅業工程, 2006(4):68 [10] Li H, Liu G Y. A novel method for the preparation of thionocarbamate. Fine Chem Intermed, 2022, 52(1): 56李華, 劉廣義. 硫代氨基甲酸酯制備新工藝研究. 精細化工中間體, 2022, 52(1):56 [11] Zhong H, Ma X, Wang S, et al. Method for Preparing Thionocarbamate and Trithiocarbonate: China Patent, 201610801951. 2017-02-08鐘宏, 馬鑫, 王帥, 等. 一種制備硫氨酯并聯產三硫代碳酸鹽的方法: 中國專利, 201610801951. 2017-02-08 [12] Zhong H, Ma X, Wang S, et al. Method for Producing Thionocarbamate and Dibenzyl Disulfide: China Patent, 201610801983. 2017-02-08鐘宏, 馬鑫, 王帥, 等. 一種制備硫氨酯并聯產二芐基二硫醚的方法: 中國專利, 201610801983. 2017-02-08 [13] Ma X, Wang S, Zhong H. Sodium benzyl trithiocarbonate synthesis and flotation performance to chalcopyrite. Chin J Nonferrous Met, 2018, 28(5): 1067馬鑫, 王帥, 鐘宏. 芐基三硫代碳酸鈉的合成及其對黃銅礦的浮選性能. 中國有色金屬學報, 2018, 28(5):1067 [14] Huang X P, Jia Y, Wang S, et al. Novel sodium O-benzythioethyl xanthate surfactant: Synthesis, DFT calculation and adsorption mechanism on chalcopyrite surface. Langmuir, 2019, 35(47): 15106 doi: 10.1021/acs.langmuir.9b03118 [15] Zhong H, Huang X P, Wang S, et al. Method for Preparing Thionocarbamates and Co-producing 2-mercaptoethanol or O-alkylthioethyl Xanthogenate: China Patent, 201810519232. 2018-09-25鐘宏, 黃小平, 王帥, 等. 一種制備硫氨酯并聯產2-巰基乙醇或O-烷硫基乙基黃原酸鹽的方法: 中國專利, 201810519232. 2018-09-25 [16] Xiao J J, Liu G Y, Zhong H. The adsorption mechanism of N-butoxypropyl-S-[2-(hydroxyimino) propyl] dithiocarbamate ester to copper minerals flotation. Int J Miner Process, 2017, 166: 53 doi: 10.1016/j.minpro.2017.07.003 [17] Andrew F P, Ajibade P A. Synthesis, characterization and anticancer studies of bis-(N-methyl-1-phenyldithiocarbamato) Cu(II), Zn(II), and Pt(II) complexes: Single crystal X-ray structure of the copper complex. J Coord Chem, 2018, 71(16-18): 2776 doi: 10.1080/00958972.2018.1489537 [18] Suyantara G P W, Hirajima T, Miki H, et al. Selective flotation of chalcopyrite and molybdenite using H2O2 oxidation method with the addition of ferrous sulfate. Miner Eng, 2018, 122: 312 doi: 10.1016/j.mineng.2018.02.005 [19] Du M M, Zhang Y Q, Hussain I, et al. Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study. Chemosphere, 2019, 233: 744 doi: 10.1016/j.chemosphere.2019.05.197 [20] Makhlouf M M, Radwan A S, Ghazal B. Experimental and DFT insights into molecular structure and optical properties of new chalcones as promising photosensitizers towards solar cell applications. Appl Surf Sci, 2018, 452: 337 doi: 10.1016/j.apsusc.2018.05.007 [21] Liu S, Xie L, Liu G Y, et al. Hetero-difunctional reagent with superior flotation performance to chalcopyrite and the associated surface interaction mechanism. Langmuir, 2019, 35(12): 4353 doi: 10.1021/acs.langmuir.9b00156 [22] Yin Z G, Hu Y H, Sun W, et al. Adsorption mechanism of 4-amino-5-mercapto-1, 2, 4-triazole as flotation reagent on chalcopyrite. Langmuir, 2018, 34(13): 4071 doi: 10.1021/acs.langmuir.7b03975 [23] Liu S, Zhong H, Liu G Y, et al. Cu(I)/Cu(II) mixed-valence surface complexes of S-[(2-hydroxyamino)-2-oxoethyl]-N, N-dibutyldithiocarbamate: Hydrophobic mechanism to malachite flotation. J Colloid Interface Sci, 2018, 512: 701 doi: 10.1016/j.jcis.2017.10.063 [24] Deng T, Yu S, Lotter N O, et al. Laboratory testwork of mixed xanthates for the raglan ore // Proceedings of Canadian Mineral Processors. Ottawa, 2010, 1: 253 [25] Liu G Y, Qiu Z H, Wang J Y, et al. Study of N-isopropoxypropyl-N’-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS. J Colloid Interface Sci, 2015, 437: 42 doi: 10.1016/j.jcis.2014.08.069 [26] Smart R S C. Surface layers in base metal sulphide flotation. Miner Eng, 1991, 4(7-11): 891 doi: 10.1016/0892-6875(91)90072-4 [27] McIntyre N S, Cook M G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal Chem, 1975, 47(13): 2208 doi: 10.1021/ac60363a034 [28] Szargan R, Schaufu? A, Ro?bach P. XPS investigation of chemical states in monolayers: Recent progress in adsorbate redox chemistry on sulphides. J Electron Spectrosc Relat Phenom, 1999, 100(1-3): 357 doi: 10.1016/S0368-2048(99)00055-9 [29] Beattie D A, Kempson I M, Fan L J, et al. Synchrotron XPS studies of collector adsorption and co-adsorption on gold and gold: Silver alloy surfaces. Int J Miner Process, 2009, 92(3-4): 162 doi: 10.1016/j.minpro.2009.03.009 [30] Acres R G, Harmer S L, Beattie D A. Synchrotron XPS, NEXAFS, and ToF-SIMS studies of solution exposed chalcopyrite and heterogeneous chalcopyrite with pyrite. Miner Eng, 2010, 23(11-13): 928 doi: 10.1016/j.mineng.2010.03.007 [31] Fairthorne G, Fornasiero D, Ralston J. Effect of oxidation on the collectorless flotation of chalcopyrite. Int J Miner Process, 1997, 49(1-2): 31 doi: 10.1016/S0301-7516(96)00039-7 -




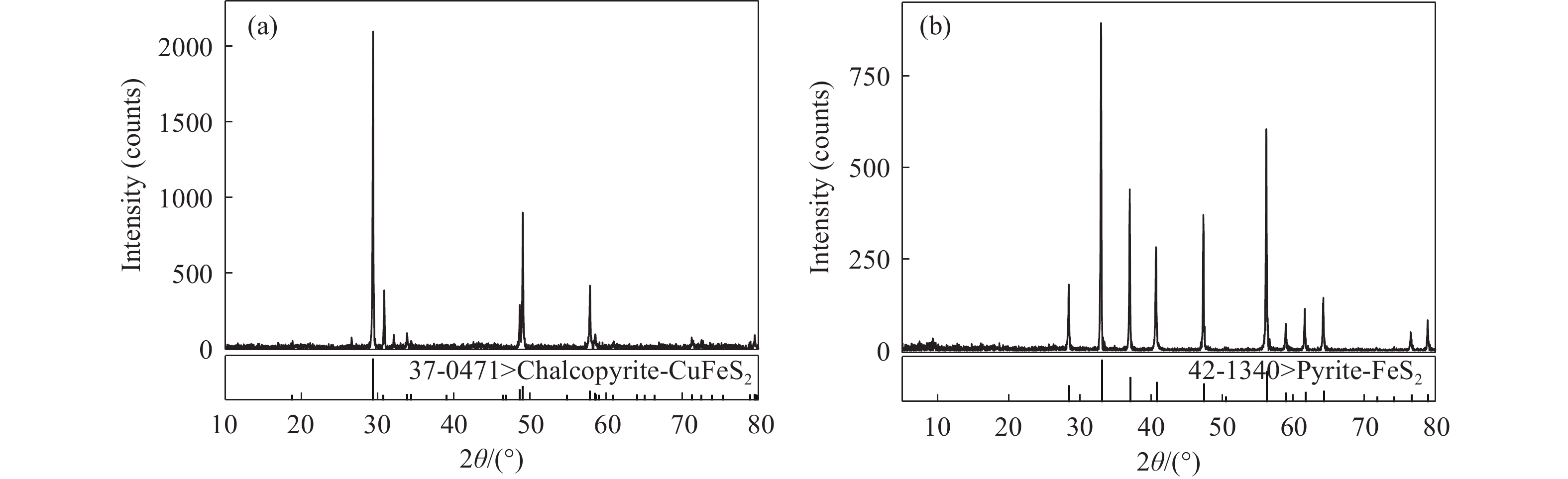
 下載:
下載: