-
摘要: 鋰離子電池因鋰資源儲量有限、分布不均及一定的安全問題,導致其在大型儲能領域的應用受限。水系鋅離子電池因其資源豐富、安全環保、易于組裝以及價格低廉等優勢在大規模儲能領域具有極大前景。但是由于鋅離子與正極材料基體具有較強的靜電吸附作用,導致其動力學緩慢以及部分正極材料在水系電解液中存在溶解等問題,限制了水系鋅離子電池的發展。在目前的正極材料中,磷酸釩鹽因其結構穩定、電壓平臺高、功率密度高等特點受到研究者的關注。然而,磷酸釩鹽作為水系鋅離子電池正極材料時,較差的電子電導率和溶解問題,制約其循環穩定性和倍率容量。本文綜述各類磷酸釩鹽及其衍生物的物相結構、合成方法、儲鋅性能和儲鋅機制,歸納提高電化學性能的方法如構建納米結構、調節電子結構、包覆導電材料、調控電解液等。最后,總結了磷酸釩鹽儲鋅正極材料現階段存在的挑戰,并對其未來的發展方向提出了展望。Abstract: With the increasing shortage of petroleum resources and serious environmental pollution, the demand for green technology development is growing stronger. Electrical energy storage is an excellent way to store intermittent clean energy and transport clean energy from one place to another. The lithium-ion battery (LIB) is broadly recognized as the first choice for electrical energy storage due to its high energy density, especially in smart electronics and electric cars. Nevertheless, the application of LIB in large-scale energy storage has been limited by various factors, including the limited and uneven distribution of lithium resources, safety issues and toxic organic electrolytes. The aqueous zinc-ion battery (AZIB) has been regarded as a potential substitute for LIB in large-scale energy storage devices because of the competitive theoretical volumetric capacity (5855 mA·h·cm?3) and gravimetric capacity (820 mA·h·g?1) of the Zn anode, the low electrochemical potential of Zn2+ (?0.76 V vs SHE), and the high ionic conductivity of the aqueous electrolyte, the ease of manufacturing (e.g., manufacture in an open-air environment), and the merits of rich resources, low cost and high safety. Finding a cathode material with high energy density and power density is proposed as a strategy to accelerate the progress of AZIB because the cathode material largely dominates the electrochemical properties and the cost of the battery. However, the strong electrostatic interaction between Zn2+ and the host material results in sluggish reaction kinetics, leading to inferior cycling performance and rate property. Some cathode materials are dissolved in aqueous electrolytes, which restrict the development of AZIB. In comparison to the reported AZIB cathodes, including vanadium-based materials, manganese-based materials, Prussian blue analogs, and organic materials, vanadium phosphates have received a lot of attention as cathodes due to their stable structures, high voltage plateaus, and high power densities. This review presents an overview of various vanadium phosphates such as Li3V2(PO4)3, Na3V2(PO4)3, VOPO4, Na3V2(PO4)2F3, NaVPO4F and their derivatives that are applied as cathodes for AZIB. The summary includes their phase structures, synthetic methods, electrochemical performance, electrochemical Zn2+ storage mechanisms and existing problems. The two major challenges in using vanadium phosphates as cathode materials for AZIB are low electronic conductivity and material dissolution problems, both of which result in inferior cycling performance and rate capacity. The resolution strategies for the mentioned challenges include designing the nanostructure, adjusting the electronic structure, coating with conductive materials, and regulating electrolytes to enhance electrochemical properties. Experimental techniques for studying electrochemical mechanisms are also proposed. Finally, the prospects for the future development of these cathodes in AZIB are advanced. It can be expected that this review has some significance for the development of new vanadium phosphates as cathode materials.
-
Key words:
- aqueous zinc-ion battery /
- cathode material /
- vanadium phosphate /
- carbon coating /
- electrolyte
-
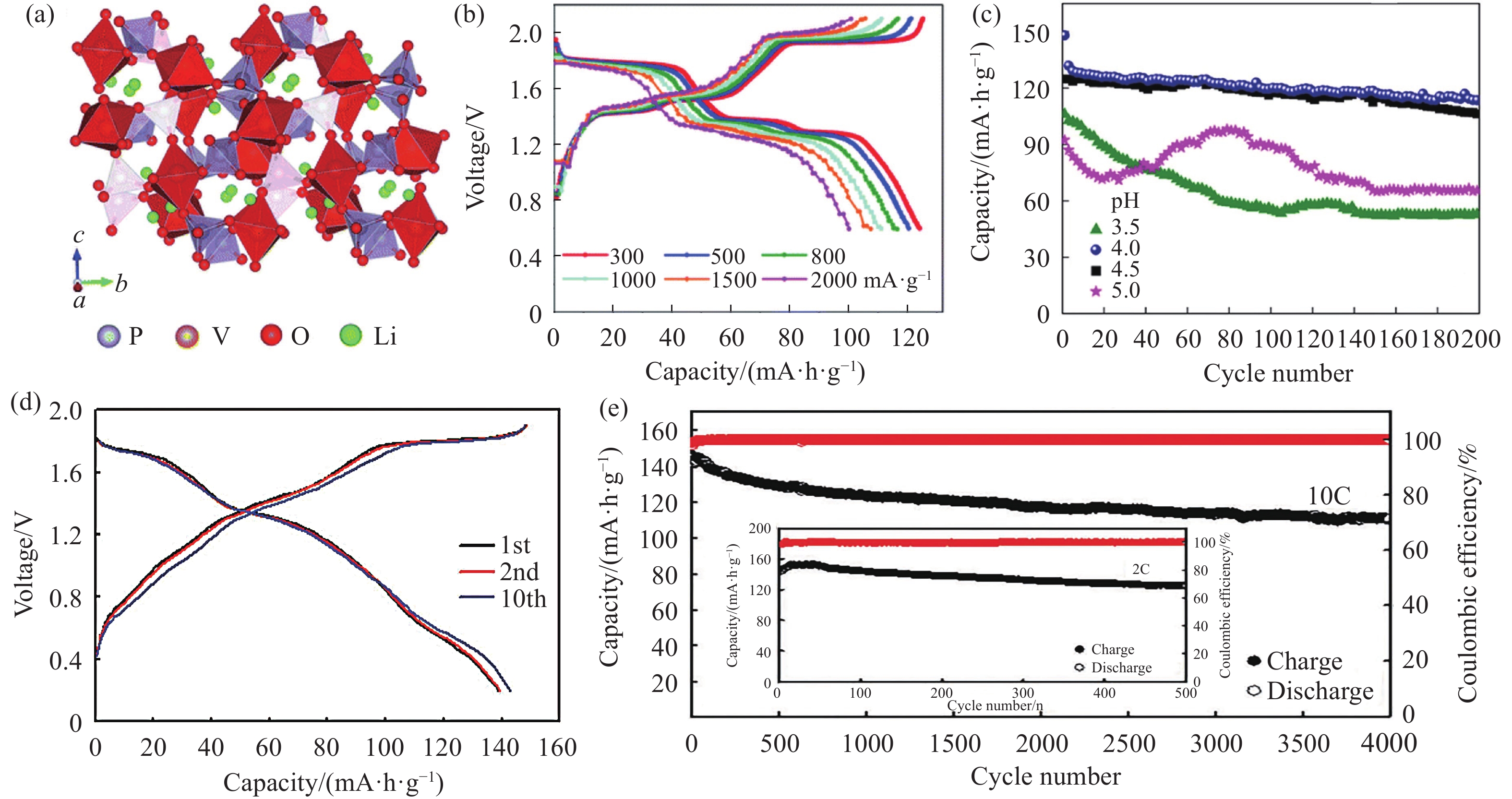
圖 1 (a) Li3V2(PO4)3的晶體結構;(b)不同電流密度下,Li3V2(PO4)3在1 mol·L?1 Zn(OTF)2+15 mol·L?1 LiTFSI電解液中的充放電曲線[34];(c) Li3V2(PO4)3@C在不同pH值電解液中0.2C循環曲線[31];(d) LiV2(PO4)3在4 mol·L?1 Zn(OTF)2電解液中2C時充放電曲線;(e) 10C長循環曲線(插圖: 2C低倍率循環曲線)[35]
Figure 1. (a) Crystal structure for Li3V2(PO4)3; (b) charge-discharge curves of Li3V2(PO4)3 at different current densities in 1 mol·L?1 Zn(OTF)2+15 mol·L?1 LiTFSI electrolyte[34]; (c) long-term cycles of Li3V2(PO4)3@C at 0.2C in various pH[31]; (d) charge-discharge curves of LiV2(PO4)3 in 4 mol·L?1 Zn(OTF)2 electrolyte at 2C; (e) cycling performance at 10C (Inset: the low rate of 2C)[35]
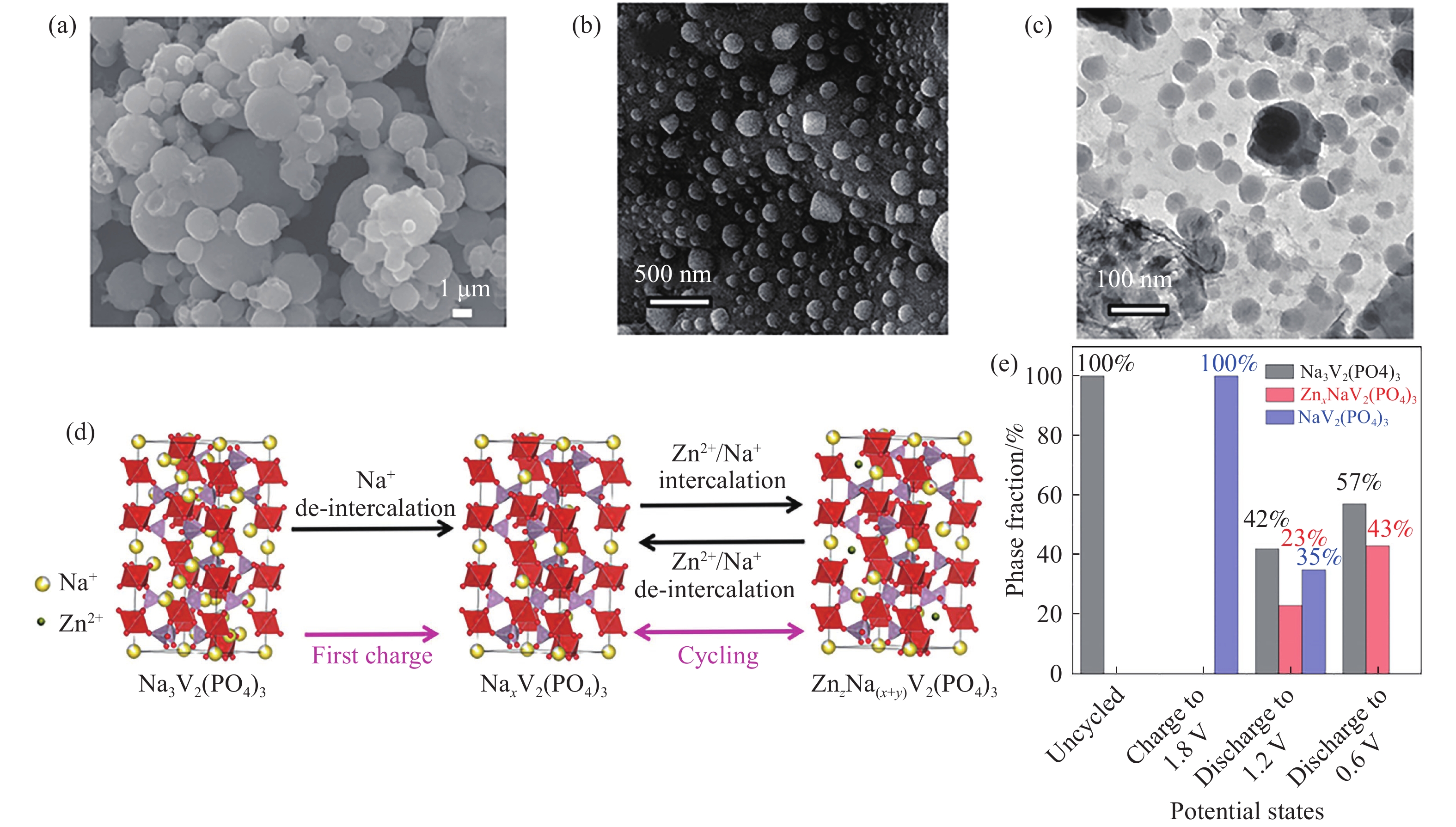
圖 2 (a) Na3V2(PO4)3@rGO的SEM圖[40];C–rGO–Na3V2(PO4)3的(b) SEM和(c) TEM圖[42];(d) Na3V2(PO4)3@rGO在充放電過程中結構演變示意圖[40]; (e)不同電壓下的Na3V2(PO4)3,NaV2(PO4)3和ZnxNaV2(PO4)3(x=0.25)物相比例[39]
Figure 2. (a) SEM image of Na3V2(PO4)3@rGO[40]; (b) SEM and (c) TEM images of C–rGO–Na3V2(PO4)3[42]; (d) schematic structure evolution of Na3V2(PO4)3@rGO during the charge/discharge process[40]; (e) phase fraction contributions of Na3V2(PO4)3, NaV2(PO4)3 and ZnxNaV2(PO4)3 (x=0.25) at different potential states[39]
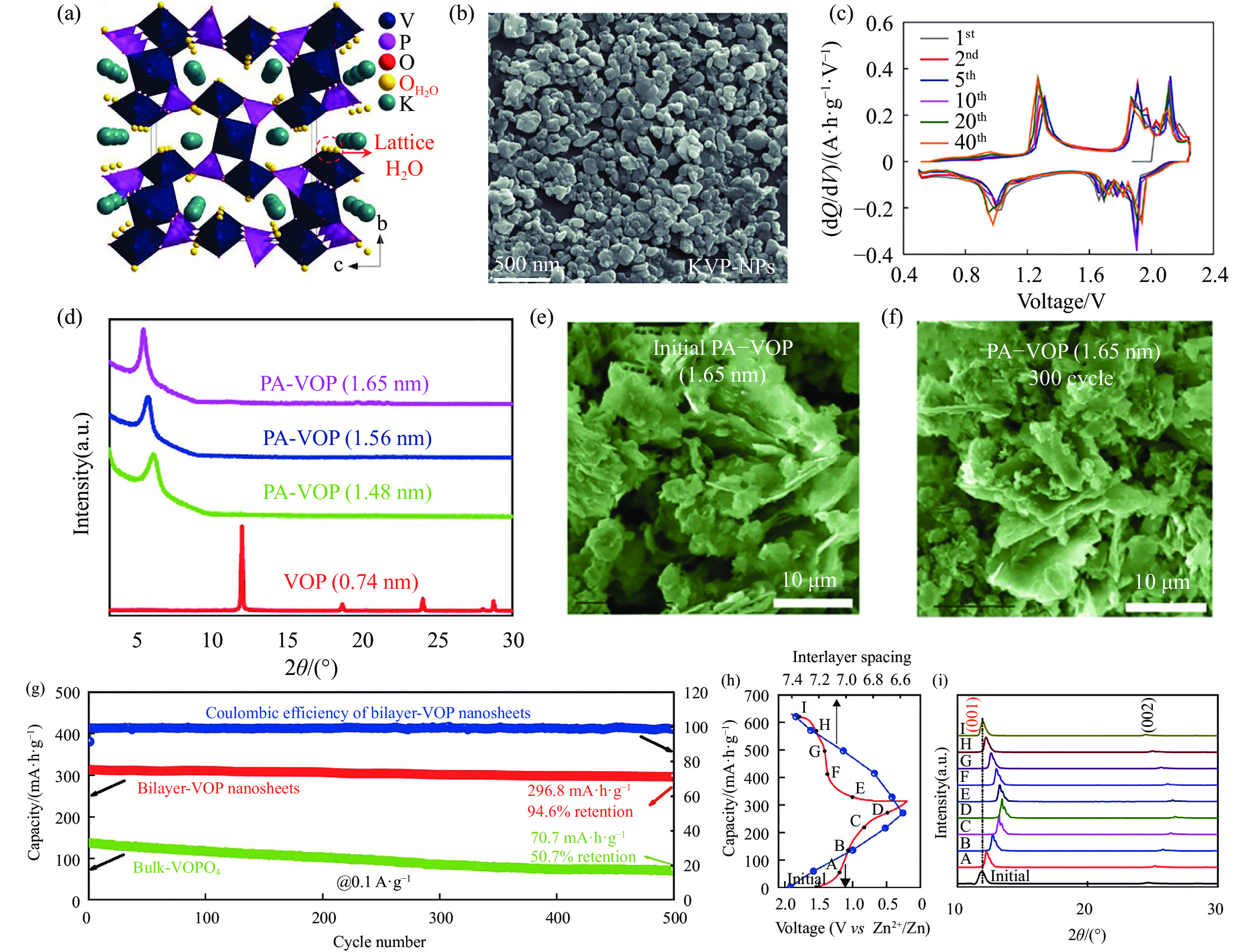
圖 3 (a) KVP的晶體結構圖;(b) KVP的SEM圖[30];(c) VOPO4·2H2O在WITS電解液的微分容量曲線[47];(d)在不同溫度和溶劑下合成的PA–VOP樣品的XRD圖;(e)PA–VOP原始電極片SEM圖;(f)循環300圈后PA–VOP電極片的SEM圖[45];(g)塊體-VOP和雙層-VOP在0.1 A·g–1電流密度下長循環曲線;雙層-VOP的(h)充放電曲線和層間距變化圖和(i)非原位XRD[27]
Figure 3. (a) Crystal structure pattern of KVP; (b) SEM image of KVP[30]; (c) differential capacity curves of VOPO4·2H2O in WITS electrolyte[47]; (d) XRD patterns of PA–VOP samples synthesized under different temperatures and solvents; (e) SEM image of PA–VOP pristine electrode; (f) SEM image of PA–VOP electrode after 300 cycles[45]; (g) cycling performance at 0.1 A·g?1 for bulk-VOP and bilayer-VOP; (h) charge–discharge curve and the d-spacing variation and (i) ex-situ XRD patterns of bilayer-VOP[27]
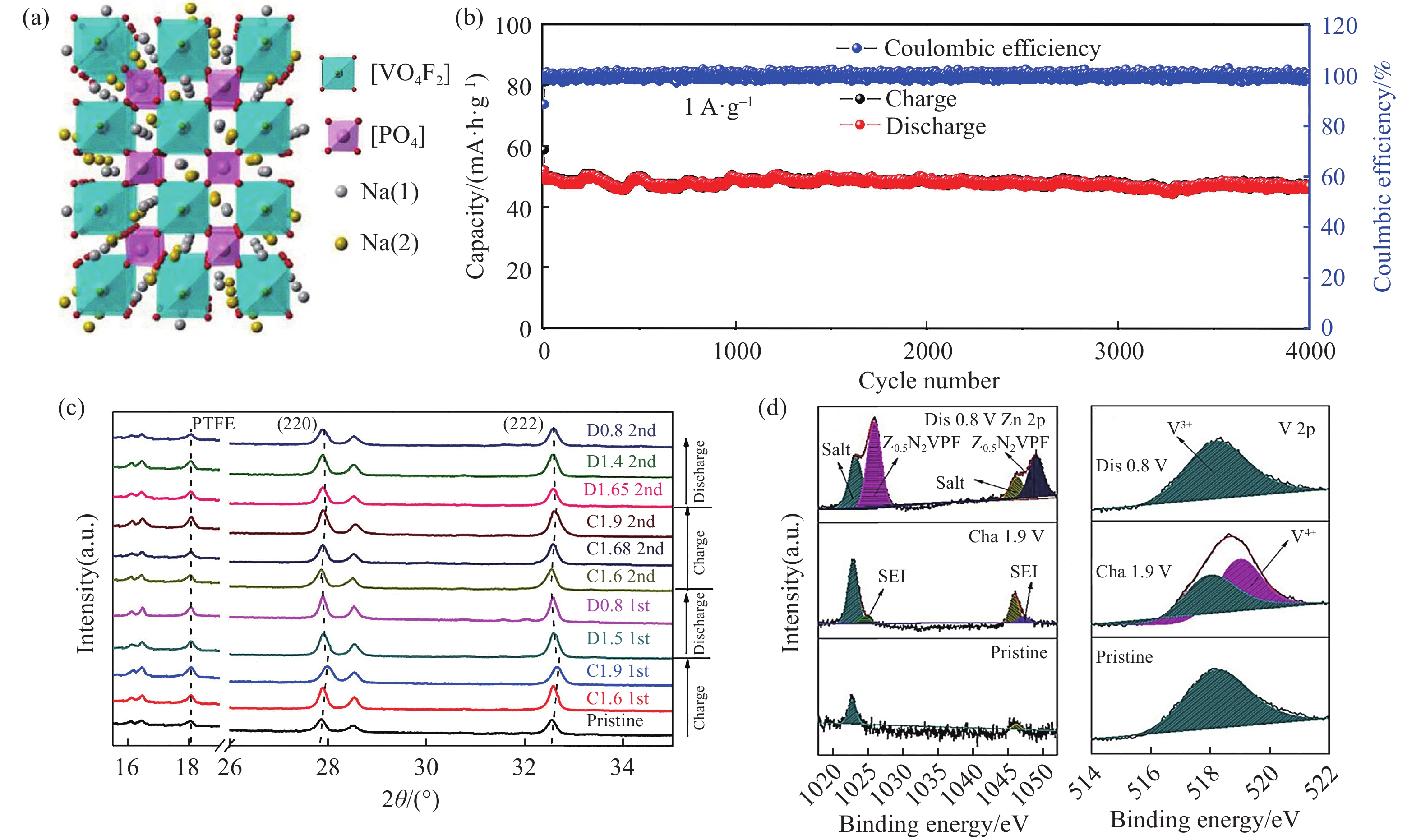
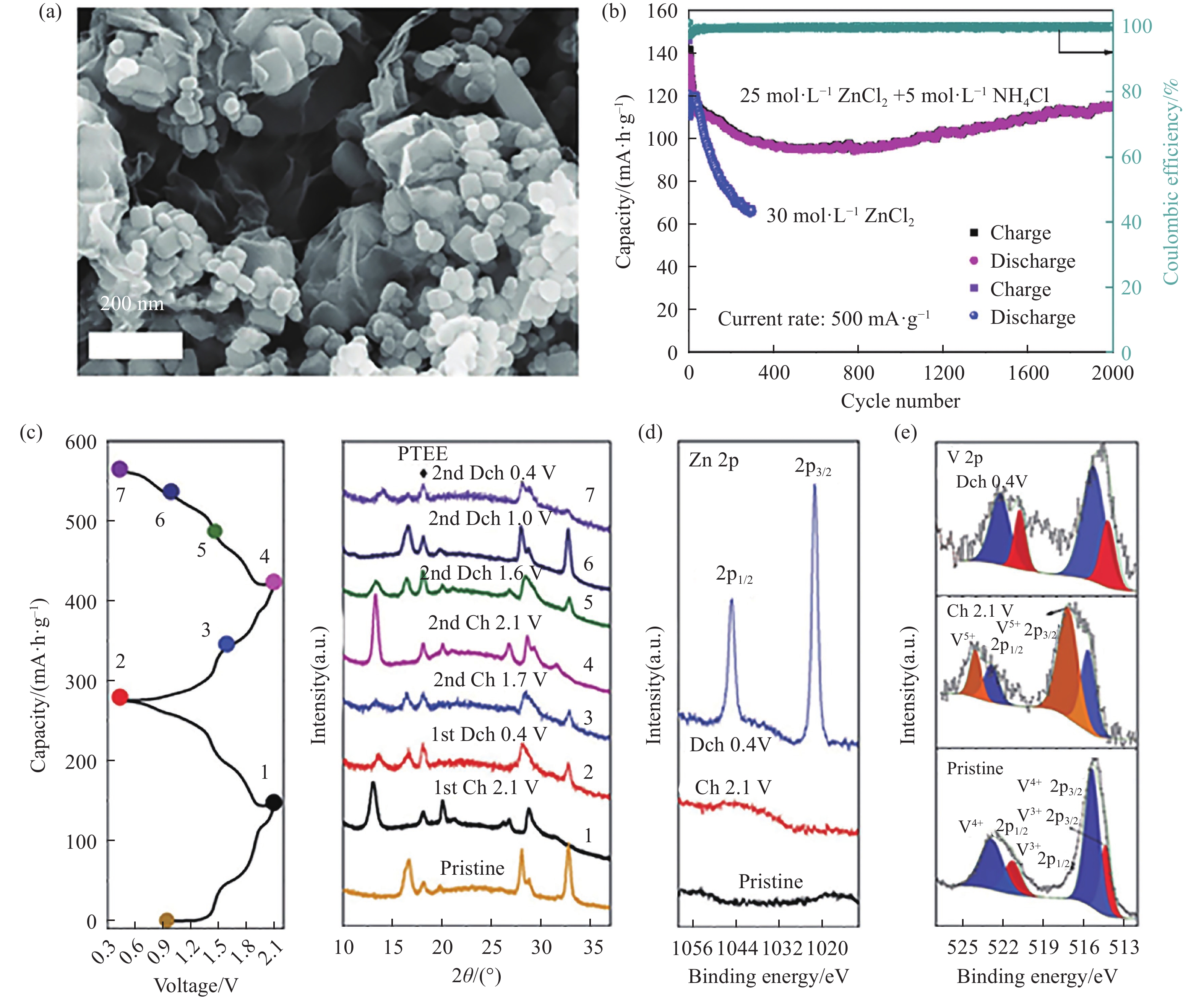
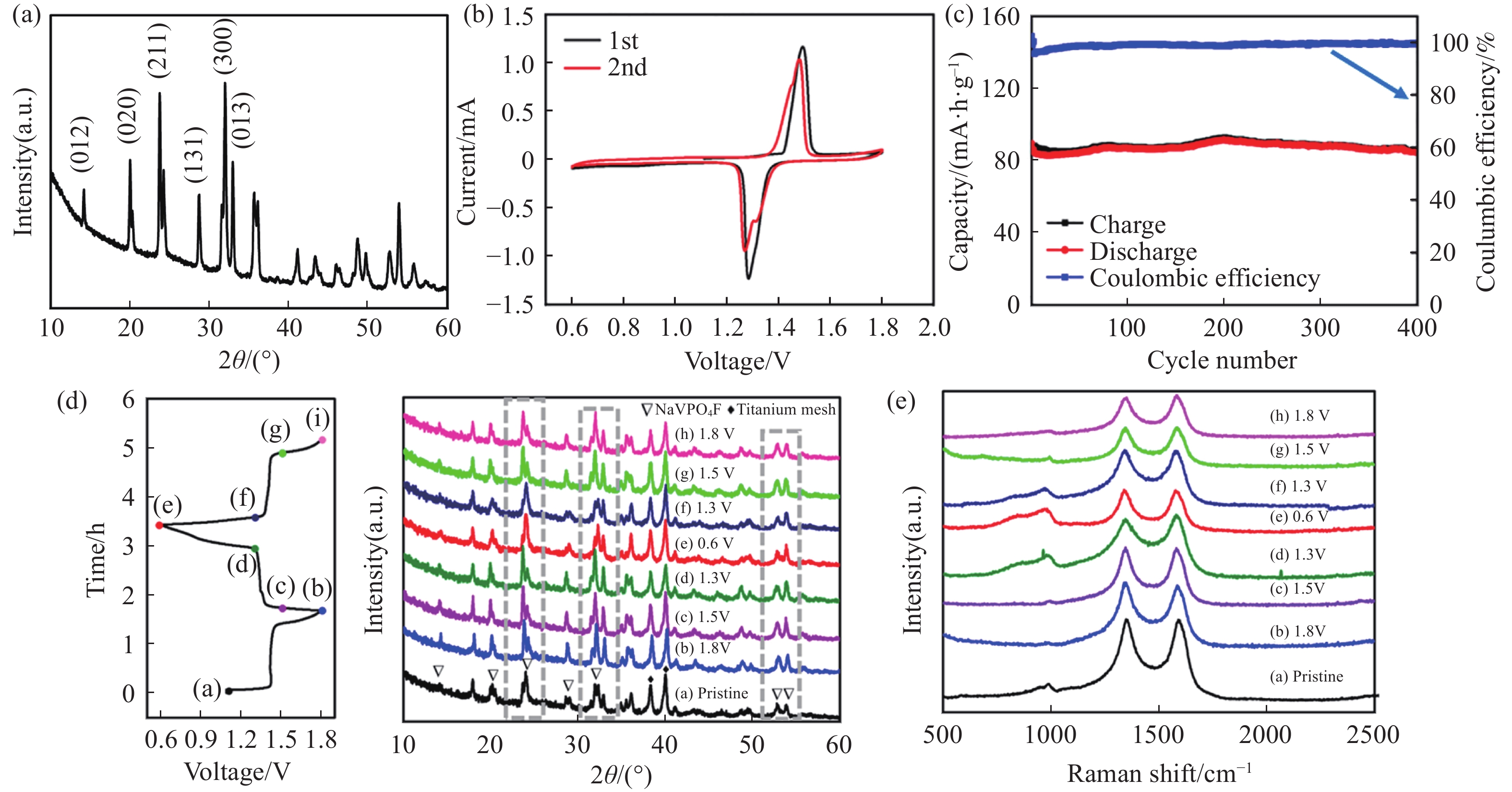
圖 5 Na3V2(PO4)2O1.6F1.4圖. (a)SEM;(b)在電解液25 mol·L?1 ZnCl2+5 mol·L?1 NH4Cl(紅)和30 mol·L?1 ZnCl2(藍)下的長循環曲線;(c)非原位XRD;(d)Zn 2p和(e)V 2p的非原位XPS[49]
Figure 5. Na3V2(PO4)2O1.6F1.4@rGO images: (a) SEM; (b) cycling performance in the 25 mol·L?1 ZnCl2+5 mol·L?1 NH4Cl (red) and 30 mol·L?1 ZnCl2 electrolyte (blue); (c) ex-situ XRD; ex-situ XPS of (d) Zn 2p and (e) V 2p[49]
表 1 水系鋅離子電池正極材料磷酸釩鹽的制備方法和電化學性能
Table 1. Preparation and performance of vanadium phosphate cathode materials for AZIB
Materials morphology Preparation Electrolyte Specific capacity/
(mA·h·g?1)Discharge plateaus/V Cycle number, n (capacity/(mA·h·g?1),
retention ratio, current density)Li3V2(PO4)3/C nanoparticles[34] Hydrothermal-assisted sol-gel 1 mol·L?1 Zn(OTF)2+15 mol·L?1 LiTFSI 126.7
(200 mA·g?1)1.75, 1.35, 1.25 2000 (89.7, 82.3%, 1000 mA·g?1) Bulk Li3V2(PO4)3@C[31] Sol-gel 1 mol·L?1 Li2SO4 + 2 mol·L?1 ZnSO4 113.5
(0.2C)1.45, 1.35 200 (96.9, 85.4%, 0.2C) LiV2(PO4)3@C microspheres[35] Spray-drying 4 mol·L?1 Zn(OTF)2 141.0
(2C)1.7, 1.2 4000 (118.2, 78.8%, 10C) Bulk Na3V2(PO4)3@C[41] Sol-gel 4 mol·L?1 Zn(OTF)2 120.3
(50 mA·g?1)1.3, 1.05 1000 (75.0, 77.8%, 2000 mA·g?1) Na3V2(PO4)3@rGO microspheres[40] Spray-drying 2 mol·L?1 Zn(OTF)2 114.0
(50 mA·g?1)1.26, 1.02 200 (74.0, 75%, 500 mA·g?1) C-rGO-Na3V2(PO4)3 nanoparticles[42] Sol-gel 0.5 mol·L?1 CH3COONa+
Zn(CH3COO)293.0
(0.5C)1.37 200 (71.6, 77%, 0.5C) KV2O4PO4·3.2H2O nanoparticles[30] Reflux 3 mol·L?1 Zn(OTF)2 228.0
(20 mA·g?1)0.93, 0.59 3000 (118.0, 75%, 3 A·g?1) VOPO4·2H2O nanosheets[47] Reflux 0.5 mol·L?1 Zn(ClO4)2+16 mol·L?1 NaClO4+3 mol·L?1 NaCF3SO3 140.0
(0.1 A·g?1)1.9, 1.7, 1.0 500 (95, 95%, 1 A·g?1) PA?VOPO4·2H2O nanosheets[45] Reflux and
solvothermal2 mol·L?1 Zn(OTF)2 268.2
(0.1 A·g?1)1.3 2000 (185.4, 92.3%, 5 A·g?1) Bilayer VOPO4·2H2O nanosheets[27] Liquid-exfoliation 2 mol·L?1 ZnSO4 313.7
(0.1 A·g?1)0.9, 0.6 2000 (158.5, 76.8%, 5 A·g?1) Bulk Na3V2(PO4)2F3@C[51] Sol-gel 2 mol·L?1 Zn(OTF)2 65.1
(0.2 A·g?1)1.6, 1.25 4000 (46.0, 95%, 0.2 A·g?1) Na3V2(PO4)2O1.6F1.4@rGO nanoparticles[49] Solvothermal 25 mol·L?1 ZnCl2+5 mol·L?1 NH4Cl 139
(500 mA·g?1)1.89, 1.47 7000 (61.0, 73.5%, 2 A·g?1) C-NaVPO4F nanoparticles[6] Sol-gel 15 mol·L?1 NaClO4+1 mol·L?1 Zn(OTF)2 89.6
(100 mA·g?1)1.31, 1.27 4000 (66.7, 89.3%, 1 A·g?1) www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Peng M K, Wang L, Li L B, et al. Molecular crowding agents engineered to make bioinspired electrolytes for high-voltage aqueous supercapacitors. eScience, 2021, 1(1): 83 doi: 10.1016/j.esci.2021.09.004 [2] Zhou T, Zhu L M, Xie L L, et al. Cathode materials for aqueous zinc-ion batteries: A mini review. J Colloid Interface Sci, 2022, 605: 828 doi: 10.1016/j.jcis.2021.07.138 [3] Xu S F, Dai H C, Zhu S L, et al. A branched dihydrophenazine-based polymer as a cathode material to achieve dual-ion batteries with high energy and power density. eScience, 2021, 1(1): 60 doi: 10.1016/j.esci.2021.08.002 [4] Dong Y, Miao L C, Ma G Q, et al. Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem Sci, 2021, 12(16): 5843 doi: 10.1039/D0SC06734B [5] Su Q S, Rong Y, Chen H Z, et al. Carbon-doped vanadium nitride used as a cathode of high-performance aqueous zinc ion batteries. Ind Eng Chem Res, 2021, 60(33): 12155 doi: 10.1021/acs.iecr.1c01915 [6] Bin D, Wang Y R, Tamirat A G, et al. Stable high-voltage aqueous zinc battery based on carbon-coated NaVPO4F cathode. ACS Sustainable Chem Eng, 2021, 9(8): 3223 doi: 10.1021/acssuschemeng.0c08651 [7] Wang X Y, Ma L W, Zhang P C, et al. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl Surf Sci, 2020, 502: 144207 doi: 10.1016/j.apsusc.2019.144207 [8] Tang B Y, Shan L T, Liang S Q, et al. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ Sci, 2019, 12(11): 3288 doi: 10.1039/C9EE02526J [9] Wan F, Zhou X Z, Lu Y, et al. Energy storage chemistry in aqueous zinc metal batteries. ACS Energy Lett, 2020, 5(11): 3569 doi: 10.1021/acsenergylett.0c02028 [10] Heng Y L, Gu Z Y, Guo J Z, et al. Research progresses on vanadium-based cathode materials for aqueous zinc-ion batteries. Acta Phys Chimica Sin, 2021, 37(3): 17衡永麗, 谷振一, 郭晉芝, 等. 水系鋅離子電池用釩基正極材料的研究進展. 物理化學學報, 2021, 37(3):17 [11] Zhai X L, Liu Y, Wang F, et al. Recent progress of vanadium-based cathode materials for rechargeable aqueous zinc-ion batteries. Chem Ind Eng, 2020, 37(5): 37翟小亮, 柳勇, 王飛, 等. 水基鋅離子電池釩基正極材料研究進展. 化學工業與工程, 2020, 37(5):37 [12] Fang G Z, Zhou J, Pan A Q, et al. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett, 2018, 3(10): 2480 doi: 10.1021/acsenergylett.8b01426 [13] Wan F, Niu Z Q. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew Chem Int Ed, 2019, 58(46): 16358 doi: 10.1002/anie.201903941 [14] Li W, Jing X Y, Jiang K, et al. Observation of structural decomposition of Na3V2(PO4)3 and Na3V2(PO4)2F3 as cathodes for aqueous Zn-ion batteries. ACS Appl Energy Mater, 2021, 4(3): 2797 doi: 10.1021/acsaem.1c00067 [15] Zhang N, Chen X Y, Yu M, et al. Materials chemistry for rechargeable zinc-ion batteries. Chem Soc Rev, 2020, 49(13): 4203 doi: 10.1039/C9CS00349E [16] Dong Y, Jia M, Wang Y Y, et al. Long-life zinc/vanadium pentoxide battery enabled by a concentrated aqueous ZnSO4 electrolyte with proton and zinc ion co-intercalation. ACS Appl Energy Mater, 2020, 3(11): 11183 doi: 10.1021/acsaem.0c02126 [17] Lin X D, Zhou G D, Liu J P, et al. Bifunctional hydrated gel electrolyte for long-cycling Zn-ion battery with NASICON-type cathode. Adv Funct Mater, 2021, 31(42): 2105717 doi: 10.1002/adfm.202105717 [18] Liu N, Li B, He Z X, et al. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries. J Energy Chem, 2021, 59: 134 doi: 10.1016/j.jechem.2020.10.044 [19] Zhang N, Cheng F Y, Liu Y C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J Am Chem Soc, 2016, 138(39): 12894 doi: 10.1021/jacs.6b05958 [20] Xu C W, Yang Z W, Zhang X K, et al. Prussian blue analogues in aqueous batteries and desalination batteries. Nanomicro Lett, 2021, 13(1): 166 [21] Liu Z, Pulletikurthi G, Endres F. A Prussian blue/zinc secondary battery with a bio-ionic liquid-water mixture as electrolyte. ACS Appl Mater Interfaces, 2016, 8(19): 12158 doi: 10.1021/acsami.6b01592 [22] Dai Y H, Gan Z W, Ruan Y S, et al. Research progress of aqueous zinc ion batteries and their key materials. J Chin Ceram Soc, 2021, 49(7): 1323戴宇航, 甘志偉, 阮雨杉, 等. 水系鋅離子電池及關鍵材料研究進展. 硅酸鹽學報, 2021, 49(7):1323 [23] Ouyang B, Wang J Y, He T J, et al. Synthetic accessibility and stability rules of NASICONs. Nat Commun, 2021, 12: 5752 doi: 10.1038/s41467-021-26006-3 [24] Wang M Y, Zhao X X, Guo J Z, et al. Enhanced electrode kinetics and properties via anionic regulation in polyanionic Na3+xV2(PO4)3?x(P2O7)x cathode material. Green Energy Environ, 2022, 7(4): 763 doi: 10.1016/j.gee.2020.11.026 [25] Liu Z X, Yang Q, Wang D H, et al. A flexible solid-state aqueous zinc hybrid battery with flat and high-voltage discharge plateau. Adv Energy Mater, 2019, 9(46): 1902473 doi: 10.1002/aenm.201902473 [26] Hu F D, Jiang X L. Superior performance of carbon modified Na3V2(PO4)2F3 cathode material for sodium-ion batteries. Inorg Chem Commun, 2021, 129: 108653 doi: 10.1016/j.inoche.2021.108653 [27] Wu Z Y, Lu C J, Ye F, et al. Bilayered VOPO4·2H2O nanosheets with high-concentration oxygen vacancies for high-performance aqueous zinc-ion batteries. Adv Funct Mater, 2021, 31(45): 2106816 doi: 10.1002/adfm.202106816 [28] Cao L S, Li D, Soto F A, et al. Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes. Angew Chem Int Ed, 2021, 60(34): 18845 doi: 10.1002/anie.202107378 [29] Li C C, Wu W L, Shi H Y, et al. The energy storage behavior of a phosphate-based cathode material in rechargeable zinc batteries. Chem Commun, 2021, 57(51): 6253 doi: 10.1039/D1CC00584G [30] Yang X, Deng W Z, Chen M, et al. Mass-producible, quasi-zero-strain, lattice-water-rich inorganic open-frameworks for ultrafast-charging and long-cycling zinc-ion batteries. Adv Mater, 2020, 32(45): 2003592 doi: 10.1002/adma.202003592 [31] Zhao H B, Hu C J, Cheng H W, et al. Novel rechargeable M3V2(PO4)3//zinc (M = Li, Na) hybrid aqueous batteries with excellent cycling performance. Sci Rep, 2016, 6: 25809 doi: 10.1038/srep25809 [32] Duan W C, Hu Z, Zhang K, et al. Li3V2(PO4)3@C core-shell nanocomposite as a superior cathode material for lithium-ion batteries. Nanoscale, 2013, 5(14): 6485 doi: 10.1039/c3nr01617j [33] S?rensen D R, Mathiesen J K, Ravnsb?k D B. Dynamic charge-discharge phase transitions in Li3V2(PO4)3 cathodes. J Power Sources, 2018, 396: 437 doi: 10.1016/j.jpowsour.2018.06.023 [34] Li C X, Yuan W T, Li C, et al. Boosting Li3V2(PO4)3 cathode stability using a concentrated aqueous electrolyte for high-voltage zinc batteries. Chem Commun, 2021, 57(35): 4319 doi: 10.1039/D0CC08115A [35] Wang F, Hu E Y, Sun W, et al. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life. Energy Environ Sci, 2018, 11(11): 3168 doi: 10.1039/C8EE01883A [36] Chen Y J, Xu Y L, Sun X F, et al. Preventing structural degradation from Na3V2(PO4)3 to V2(PO4)3: F-doped Na3V2(PO4)3/C cathode composite with stable lifetime for sodium ion batteries. J Power Sources, 2018, 378: 423 doi: 10.1016/j.jpowsour.2017.12.073 [37] Park M J, Yaghoobnejad Asl H, Therese S, et al. Structural impact of Zn-insertion into monoclinic V2(PO4)3: Implications for Zn-ion batteries. J Mater Chem A, 2019, 7(12): 7159 doi: 10.1039/C9TA00716D [38] Chen M Z, Hua W B, Xiao J, et al. Development and investigation of a NASICON-type high-voltage cathode material for high-power sodium-ion batteries. Angew Chem Int Ed, 2020, 59(6): 2449 doi: 10.1002/anie.201912964 [39] Ko J S, Paul P P, Wan G, et al. NASICON Na3V2(PO4)3 enables quasi-two-stage Na+ and Zn2+ intercalation for multivalent zinc batteries. Chem Mater, 2020, 32(7): 3028 doi: 10.1021/acs.chemmater.0c00004 [40] Hu P, Zhu T, Wang X P, et al. Aqueous Zn//Zn(CF3SO3)2//Na3V2(PO4)3 batteries with simultaneous Zn2+/Na+ intercalation/de-intercalation. Nano Energy, 2019, 58: 492 doi: 10.1016/j.nanoen.2019.01.068 [41] Heng Y L, Gu Z Y, Guo J Z, et al. Na3V2(PO4)3@C cathode material for aqueous zinc-ion batteries. Energy Storage Sci Technol, 2021, 10(3): 938衡永麗, 谷振一, 郭晉芝, 等. Na3V2(PO4)3@C用作水系鋅離子電池正極材料的研究. 儲能科學與技術, 2021, 10(3):938 [42] Li G L, Yang Z, Jiang Y, et al. Hybrid aqueous battery based on Na3V2(PO4)3/C cathode and zinc anode for potential large-scale energy storage. J Power Sources, 2016, 308: 52 doi: 10.1016/j.jpowsour.2016.01.058 [43] Yahmed S E, Ayed M, Zid M F, et al. β-K(VO2)2(PO4). Acta Cryst, 2013, E69: i2 [44] Xiong P, Zhang F, Zhang X Y, et al. Strain engineering of two-dimensional multilayered heterostructures for beyond-lithium-based rechargeable batteries. Nat Commun, 2020, 11(1): 3297 doi: 10.1038/s41467-020-17014-w [45] Hu L F, Wu Z Y, Lu C J, et al. Principles of interlayer-spacing regulation of layered vanadium phosphates for superior zinc-ion batteries. Energy Environ Sci, 2021, 14(7): 4095 doi: 10.1039/D1EE01158H [46] Shi H Y, Song Y, Qin Z M, et al. Inhibiting VOPO4·xH2O decomposition and dissolution in rechargeable aqueous zinc batteries to promote voltage and capacity stabilities. Angew Chem Int Ed, 2019, 58(45): 16057 doi: 10.1002/anie.201908853 [47] Shi H Y, Wu W L, Yang X P, et al. Accessing the 2 V VV/VIV redox process of vanadyl phosphate cathode for aqueous batteries. J Power Sources, 2021, 507: 230270 doi: 10.1016/j.jpowsour.2021.230270 [48] Park M J, Manthiram A. Unveiling the charge storage mechanism in nonaqueous and aqueous Zn/Na3V2(PO4)2F3 batteries. ACS Appl Energy Mater, 2020, 3(5): 5015 doi: 10.1021/acsaem.0c00505 [49] Ni Q, Jiang H, Sandstrom S, et al. A Na3V2(PO4)2O1.6F1. 4 cathode of Zn-ion battery enabled by a water-in-bisalt electrolyte. Adv Funct Mater, 2020, 30(36): 2003511 doi: 10.1002/adfm.202003511 [50] Jamesh M I, Prakash A S. Advancement of technology towards developing Na-ion batteries. J Power Sources, 2018, 378: 268 doi: 10.1016/j.jpowsour.2017.12.053 [51] Li W, Wang K L, Cheng S J, et al. A long-life aqueous Zn-ion battery based on Na3V2(PO4)2F3 cathode. Energy Storage Mater, 2018, 15: 14 doi: 10.1016/j.ensm.2018.03.003 [52] Gu Z Y, Guo J Z, Cao J M, et al. An advanced high-entropy fluorophosphate cathode for sodium-ion batteries with increased working voltage and energy density. Adv Mater, 2022, 34(14): 2110108 doi: 10.1002/adma.202110108 [53] Ling M X, Lv Z Q, Li F, et al. Revisiting of tetragonal NaVPO4F: A high energy density cathode for sodium-ion batteries. ACS Appl Mater Interfaces, 2020, 12(27): 30510 doi: 10.1021/acsami.0c08846 [54] Jin T, Liu Y C, Li Y, et al. Electrospun NaVPO4F/C nanofibers as self-standing cathode material for ultralong cycle life Na-ion batteries. Adv Energy Mater, 2017, 7(15): 1700087 doi: 10.1002/aenm.201700087 [55] Mamoor M, Lian R Q, Wang D S, et al. Structure, charge transfer, and kinetic properties of NaVPO4F with Na+ extraction: A comprehensive first-principles study. Phys Chem Chem Phys, 2019, 21(27): 14612 doi: 10.1039/C9CP01819K -




 下載:
下載: