-
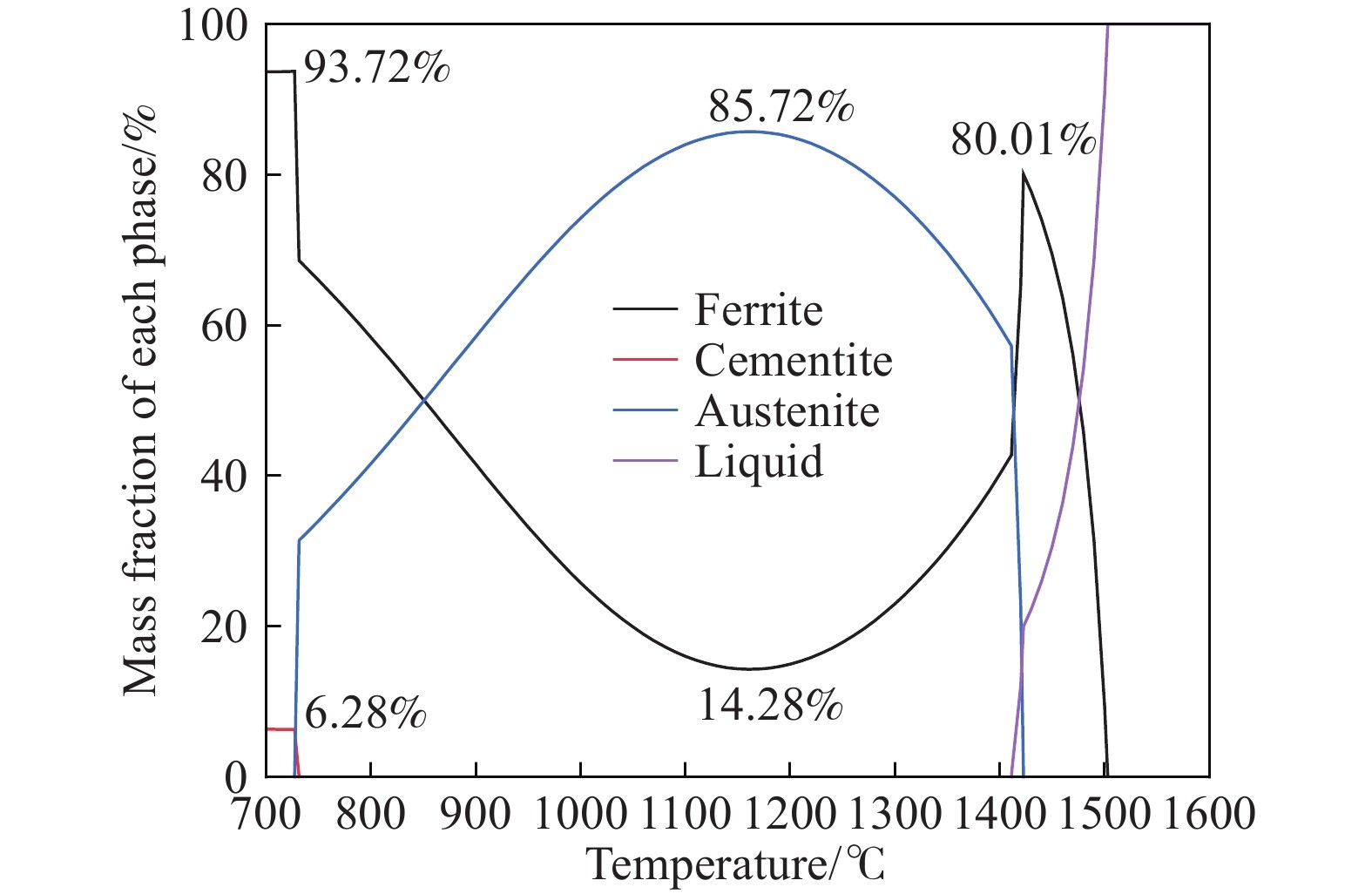
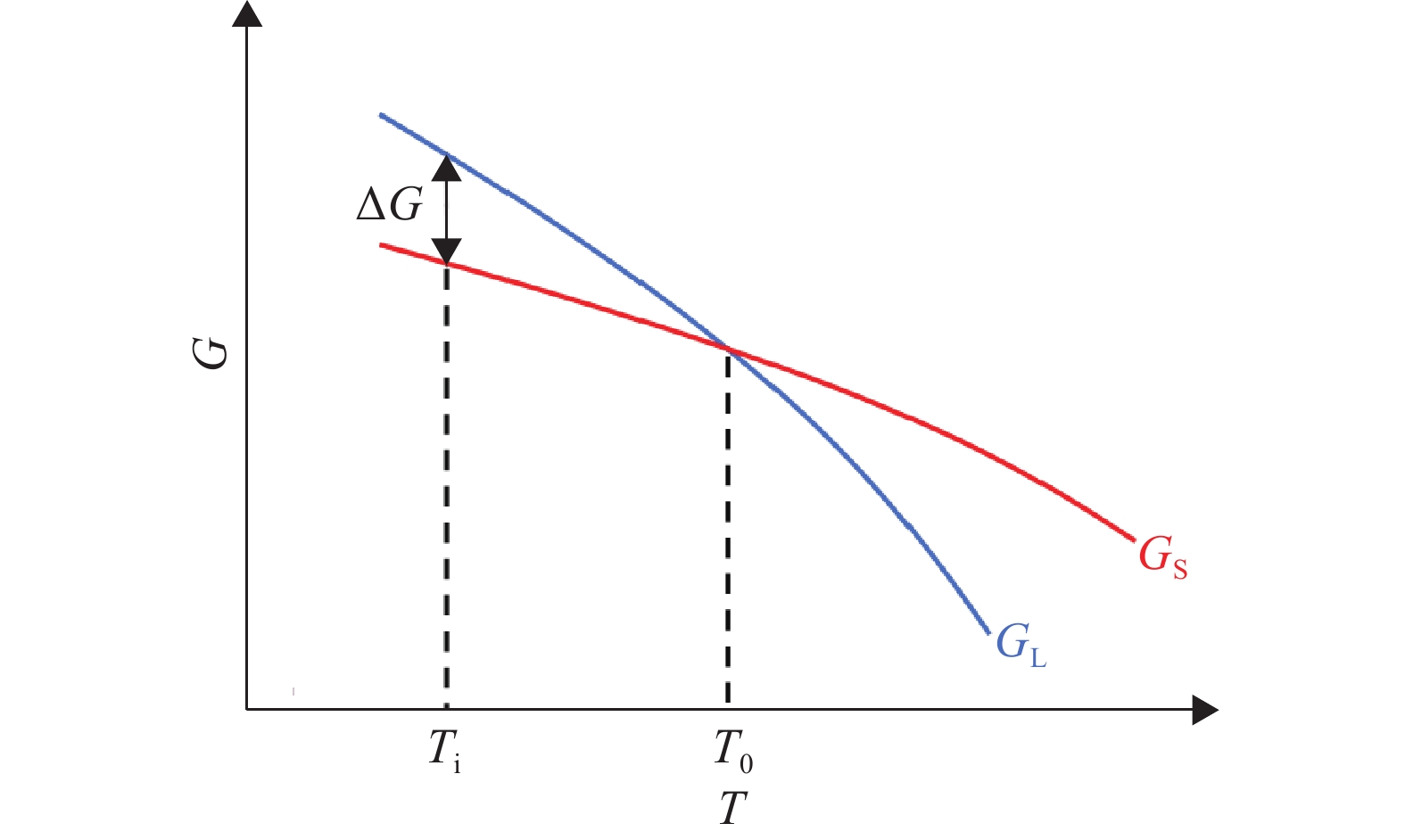
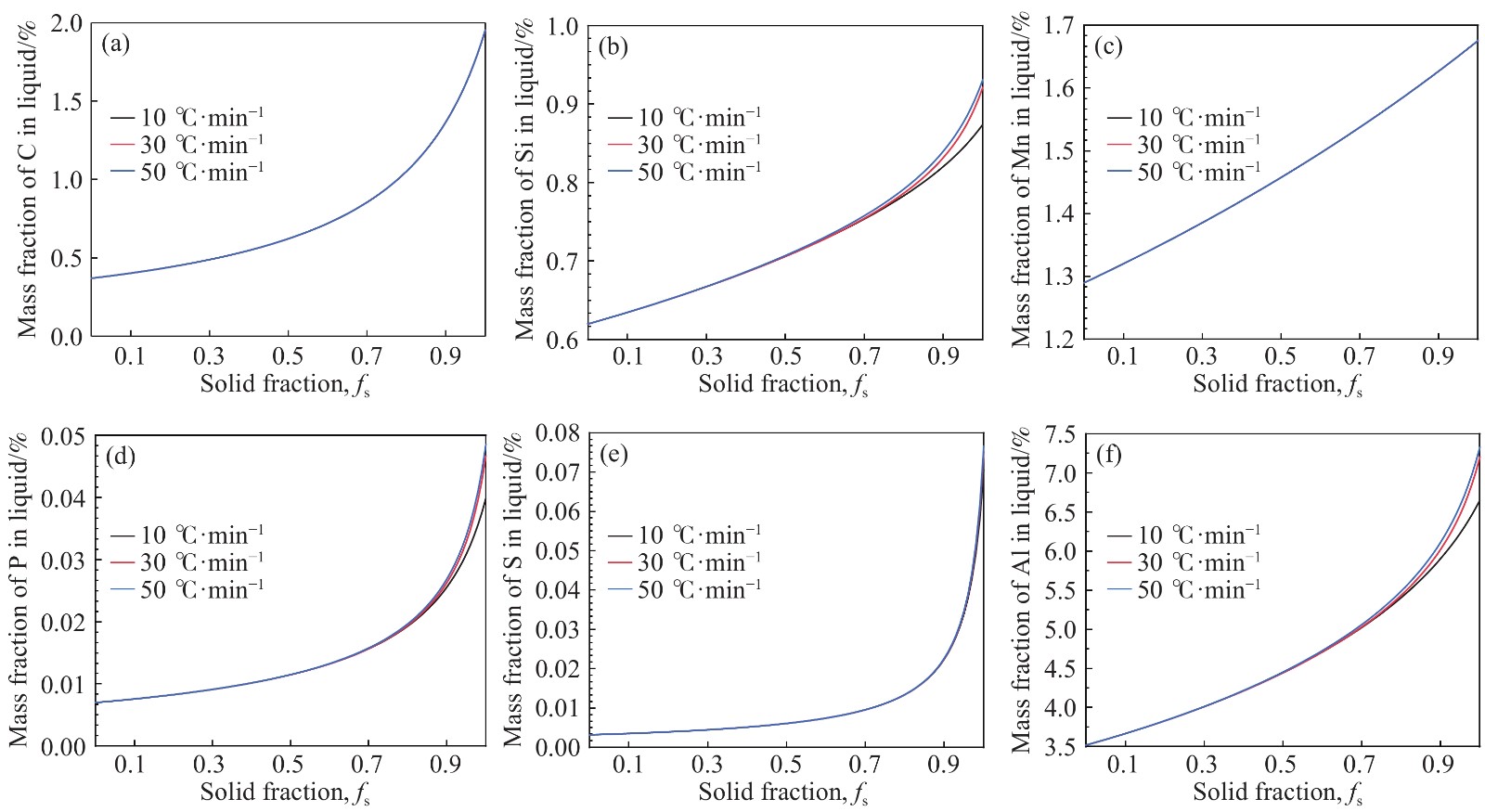
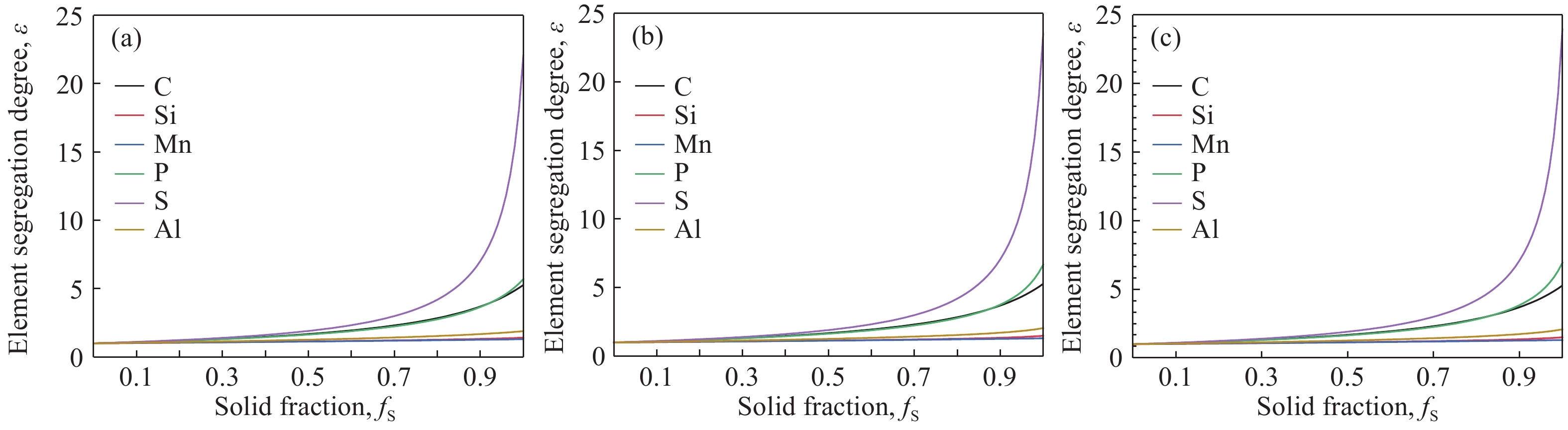
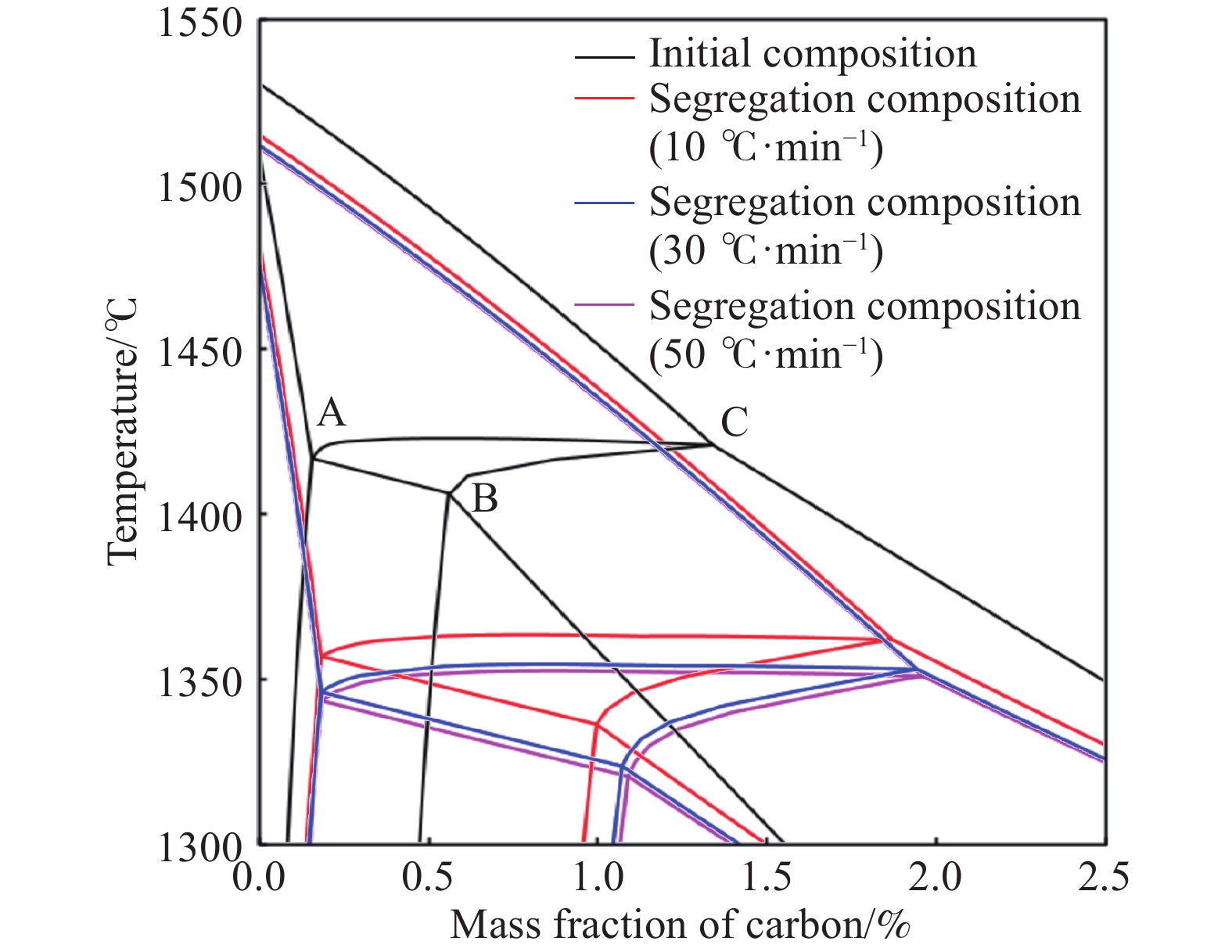
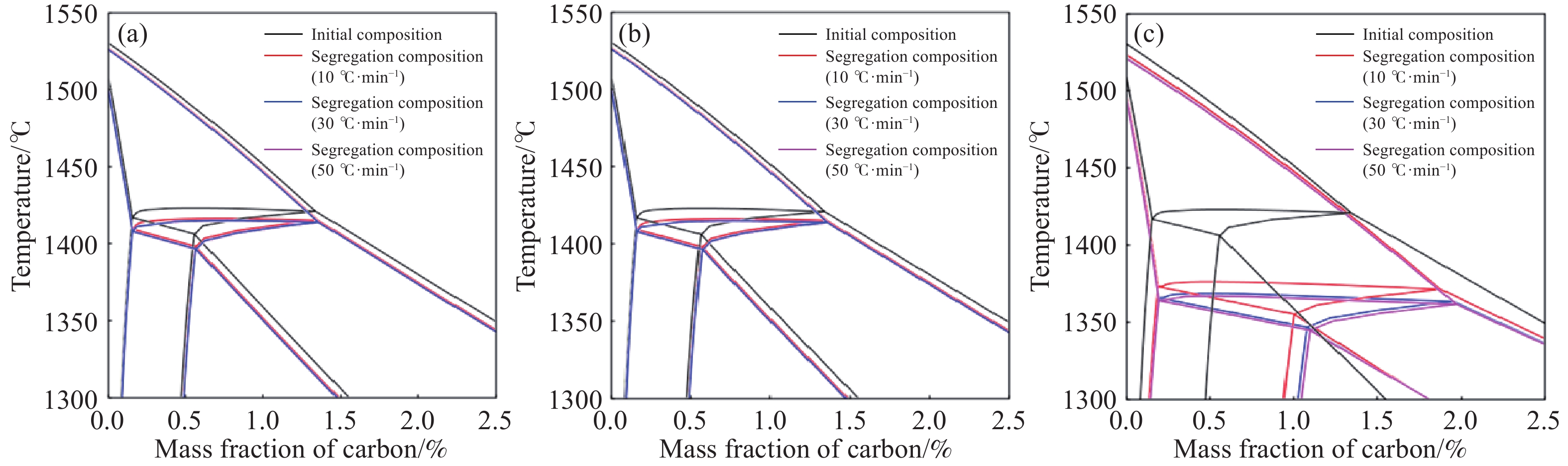
摘要: 在鋼的凝固過程中冷卻速率對鋼的相變具有不可忽視的影響。本研究采用Thermo-calc熱力學軟件,模擬計算了含Al 3.52%(質量分數)的δ鐵素體相變誘導塑性(δ-TRIP)鋼的相轉變過程,并分別使用差示掃描量熱法(DSC)和Ohnaka微觀偏析模型,分析了不同冷卻速率對3.52%Al δ-TRIP鋼凝固過程中的包晶相變溫度,以及溶質元素偏析的影響。結果表明,冷卻速率越小,DSC試驗所得的相變溫度越接近Thermo-calc計算的熱力學平衡值。隨著冷卻速率從10、30增加到50 ℃·min–1,L→L+δ的轉變溫度降低,L+δ→L+δ+γ和L+δ+γ→δ+γ的轉變溫度先降低后升高,前者主要受過冷度的影響,后者主要受元素偏析的影響。冷卻速率對C、Mn、S的偏析影響很小,對Si、P、Al的偏析影響較大,并且隨著冷卻速率的增加,Si、P、Al偏析程度增加。Si和P的偏析會小幅度延緩包晶反應的進程;Al對改變包晶反應進程作用明顯,隨著冷卻速率的增加,包晶反應區域逐漸下移,且下移趨勢漸緩。Abstract: The phase transformation of carbon steel has always been a research hotspot. Researchers study the phase transformation process of steel in terms of the original structure, chemical composition, and process conditions, and the cooling rate in process conditions has an important influence on the phase transformation of steel. In this study, Thermo-calc thermodynamic software is used to simulate and calculate the phase transformation process of 3.52%Al (mass fraction) delta ferrite transformation-induced plasticity (δ-TRIP) steel, and differential scanning calorimetry (DSC) and the Ohnaka microsegregation model are used to analyze the effect of cooling rate on the peritectic transformation temperature and solute element segregation during solidification of 3.52%Al δ-TRIP steel. The results show that the smaller the cooling rate is, the closer the DSC phase transition temperature is to the thermodynamic equilibrium value calculated by Thermo-calc. Increasing the cooling rate from 10 to 30 to 50 ℃·min?1 decreases the phase transition temperature of L→L+δ and first decreases and then increases those of L+δ→L+δ+γ and L+δ+γ→δ+γ. The former temperature is mainly affected by cooling, and the latter temperatures are mainly affected by element segregation. Among the six elements (C, Si, Mn, P, S, and Al) of 3.52%Al δ-TRIP steel, the segregation of S is the most severe. This result is obtained because the partition coefficient k of the S element at the solid–liquid interface is much smaller than those of other solute elements. The rapid S element enrichment at the end of solidification increases the possibility of sulfide precipitation, forms a low melting point liquid film between dendrites, reduces the zero plastic temperature, and increases the solidification brittleness range and crack sensitivity. Therefore, the sulfur content in steel should be strictly controlled during composition smelting. The cooling rate slightly affects C, Mn, and S segregation but greatly affects Si, P, and Al segregation, and the degree of segregation of Si, P, and Al increases with the cooling rate. The segregation of Si, P, and Al delays the peritectic reaction process, the segregation of Si and P slightly delays the peritectic reaction process, and Al segregation clearly delays the peritectic reaction process. With increasing cooling rate, the lower the peritectic reaction area moves, the slower the peritectic reaction process. This study can provide a theoretical basis for the continuous casting process parameters of δ-TRIP steel.
-
表 1 δ-TRIP鋼的化學成分(質量分數)
Table 1. Chemical composition of δ-TRIP steel
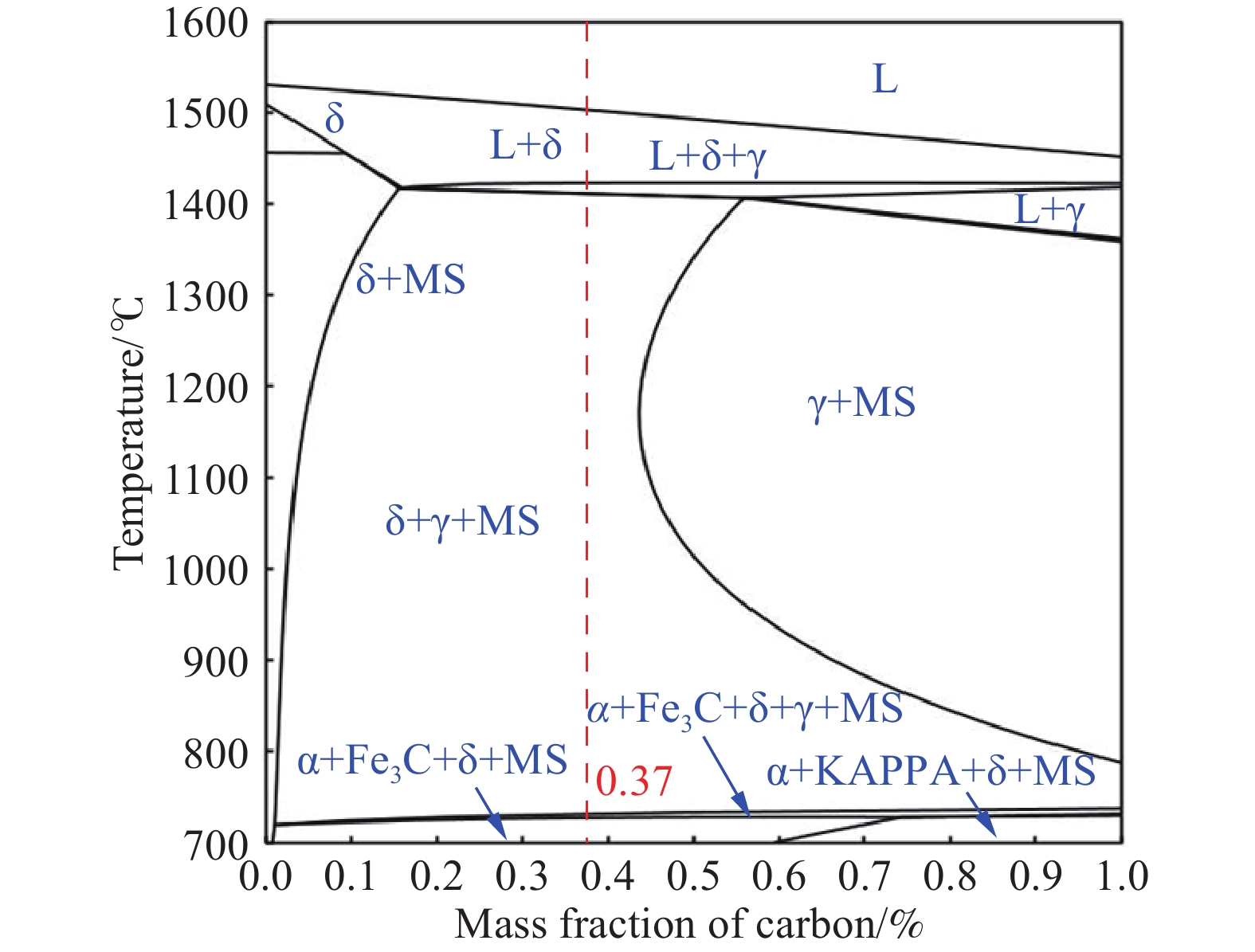
% C Si Mn P S O N Al Fe 0.37 0.62 1.29 0.0070 0.0032 0.00066 0.00099 3.52 Bal. 表 2 不同冷卻速率的DSC曲線上的點對應的溫度
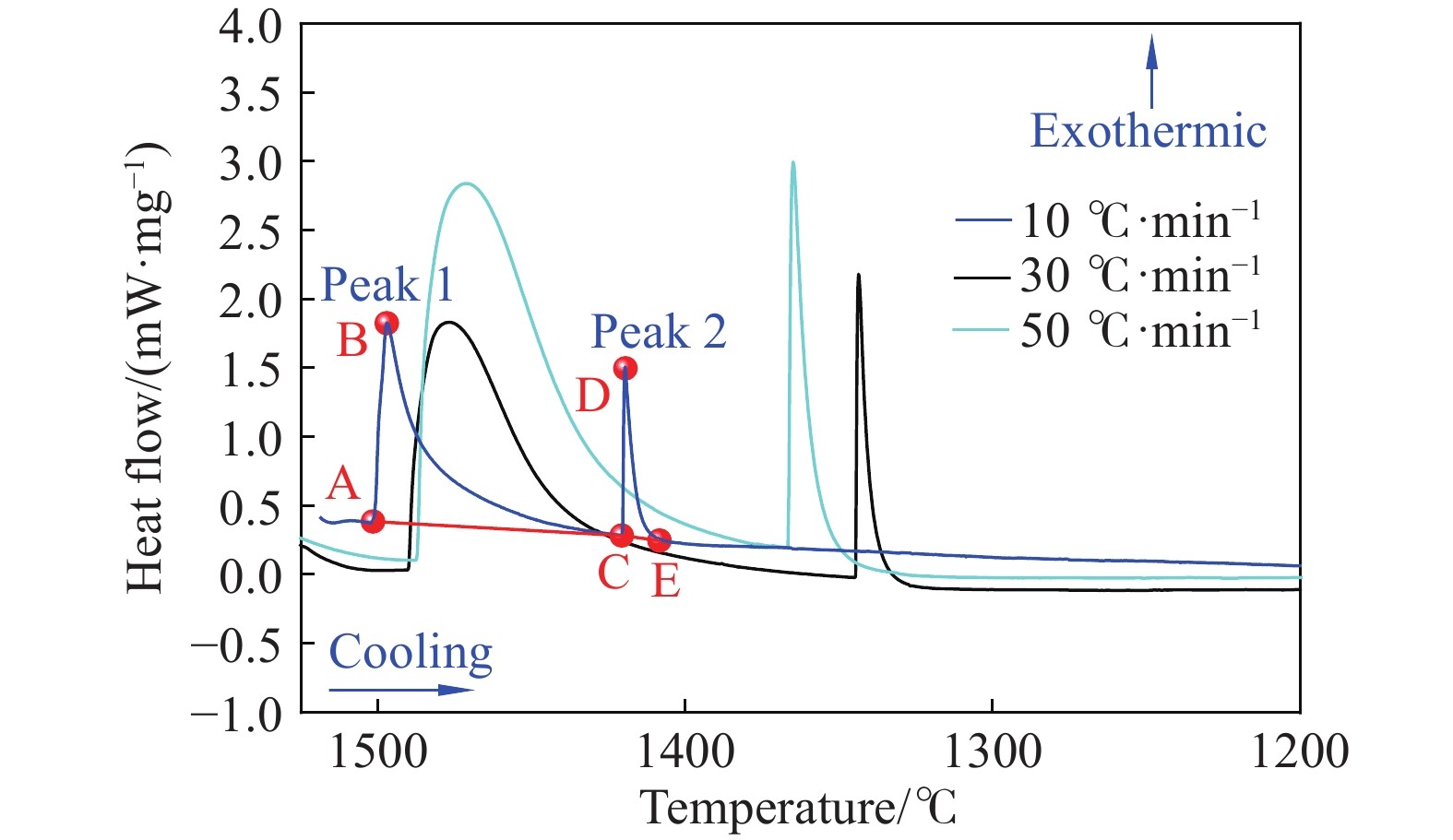
Table 2. Temperature corresponding to the points on the differential scanning calorimetry curve at different cooling rates ℃
Cooling rate/(℃·min?1) A B C D E 10 1501.0 1497.1 1420.6 1419.6 1404.8 30 1490.3 1476.9 1344.6 1343.6 1326.6 50 1487.4 1471.3 1367.0 1365.0 1336.9 表 3 不同冷卻速率下的相轉變溫度
Table 3. Phase transition temperature at different cooling rates
℃ Phase
transformation0 ℃·min?1
(Thermo-calc)10 ℃·min?1
(DSC)30 ℃·min?1
(DSC)50 ℃·min?1
(DSC)L→L+δ 1503.2 1501.0 1490.3 1487.4 L+δ→L+δ+γ 1422.7 1420.6 1344.6 1367.0 L+δ+γ→δ+γ 1411.2 1404.8 1326.6 1336.9 表 4 不同冷卻速率下各個放熱峰單位質量的相變焓值
Table 4. Phase change enthalpy per unit mass of each exothermic peak at different cooling rates J·g?1
Cooling rate/(℃·min?1) Peak 1 Peak 2 10 0.0418 0.0068 30 0.0407 0.0048 50 0.0405 0.0055 Elements k DS/(cm2·s?1) C 0.19 0.0127Exp[–81301/(RT)] Si 0.77 8.0Exp[–248710/(RT)] Mn 0.77 0.76Exp[–116935/(RT)] P 0.23 2.9Exp[–229900/(RT)] S 0.05 4.56Exp[–214434/(RT)] Al 0.6 5.9Exp[–241186/(RT)] Note: R—8.314 J·K?1?mol?1; T—Temperature in Kelvin. 表 6 DSC試驗得到的不同冷卻速率下的TL和TS
Table 6. TL and TS under different cooling rates obtained from differential scanning calorimetry experiments ℃
Cooling rate/(℃·min?1) TL TS 10 1501.0 1404.8 30 1490.3 1326.6 50 1487.4 1336.9 表 7 不同冷卻速率下各元素偏析后的最終成分(質量分數)
Table 7. Final composition of elements after segregation at different cooling rates %
Cooling rate/(℃·min?1) C Si Mn P S Al 10 1.95 0.87 1.68 0.040 0.075 6.65 30 1.95 0.92 1.68 0.047 0.075 7.20 50 1.95 0.93 1.68 0.049 0.075 7.33 www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Ma M T, Yi H L, Lu H Z, et al. On the lightweighting of automobile. Eng Sci, 2009, 11(9): 20 doi: 10.3969/j.issn.1009-1742.2009.09.004馬鳴圖, 易紅亮, 路洪洲, 等. 論汽車輕量化. 中國工程科學, 2009, 11(9):20 doi: 10.3969/j.issn.1009-1742.2009.09.004 [2] Yang Q, Wang J F, Cong Y, et al. State of knowledge on lightweight steels(Part Ⅰ)—Al-rich interstitial-free steels and ferritic lightweight steels. Baosteel Technol, 2015(3): 1 doi: 10.3969/j.issn.1008-0716.2015.03.001楊旗, 王俊峰, 叢郁, 等. 輕質鋼的研究進展(一)——富Al無間隙原子鋼和鐵素體輕質鋼. 寶鋼技術, 2015(3):1 doi: 10.3969/j.issn.1008-0716.2015.03.001 [3] Yi H L, Chen P, Wang G D, et al. δ-TRIP steel: Physical and mechanical metallurgy. Eng Sci, 2014, 16(2): 18 doi: 10.3969/j.issn.1009-1742.2014.02.002易紅亮, 陳蓬, 王國棟, 等. δ-TRIP鋼的物理與力學冶金. 中國工程科學, 2014, 16(2):18 doi: 10.3969/j.issn.1009-1742.2014.02.002 [4] Chatterjee S, Murugananth M, Bhadeshia H K D H. δTRIP steel. Mater Sci Technol, 2007, 23(7): 819 doi: 10.1179/174328407X179746 [5] Xie L, Huang T L, Wang Y H, et al. Deformation induced martensitic transformation and its initial microstructure dependence in a high alloyed duplex stainless steel. Steel Res Int, 2017, 88(12): n/a [6] Jamei F, Mirzadeh H, Zamani M. Synergistic effects of holding time at intercritical annealing temperature and initial microstructure on the mechanical properties of dual phase steel. Mater Sci Eng A, 2019, 750: 125 doi: 10.1016/j.msea.2019.02.052 [7] Liang J T, Zhao Z Z, Liu K, et al. Microstructure and properties of 1300-MPa grade Nb microalloying DH steel. Chin J Eng, 2021, 43(3): 392梁江濤, 趙征志, 劉錕, 等. 1300 MPa級Nb微合金化DH鋼的組織性能. 工程科學學報, 2021, 43(3):392 [8] Zhang Y G, Zhao A M, Zhao Z Z, et al. Influence of alloying element on continuous cooling solid phase transformation and hardness of TRIP steels. J Shenyang Univ Technol, 2010, 32(4): 390張宇光, 趙愛民, 趙征志, 等. 合金元素對TRIP鋼連續冷卻固態相變和硬度的影響. 沈陽工業大學學報, 2010, 32(4):390 [9] Bocharova E, Khlopkov K, Sebald R. Effect of microalloying elements on phase transformation, microstructure and mechanical properties in dual-phase steels. Mater Sci Forum, 2016, 879: 483 doi: 10.4028/www.scientific.net/MSF.879.483 [10] Chen Y L, Dong C, Jiang H T, et al. Effect of aluminum and silicon on phase transformation and microstructure of quenching and partitioning steel during continuous cooling. Hot Work Technol, 2010, 39(2): 10 doi: 10.3969/j.issn.1001-3814.2010.02.004陳雨來, 董辰, 江海濤, 等. Si、Al元素對QP鋼連續冷卻的相變及組織影響. 熱加工工藝, 2010, 39(2):10 doi: 10.3969/j.issn.1001-3814.2010.02.004 [11] Li D Z, Yan Z J, Wang R, et al. Effect of hot deformation processes on phase transformation of low-alloyed, multiphase, high-strength steel. Steel Res Int, 2020, 92(1): 1900522 [12] Rodrigues D G, Maria G G B, Viana N A L, et al. Effect of low cold-rolling strain on microstructure, texture, phase transformation, and mechanical properties of 2304 lean duplex stainless steel. Mater Charact, 2019, 150: 138 doi: 10.1016/j.matchar.2019.02.011 [13] Zhu J, Liu S, Jia W P, et al. Effect of annealing process on microstructure and properties of Ti+Nb-IF steel. Hot Work Technol, 2008, 37(22): 59 doi: 10.3969/j.issn.1001-3814.2008.22.019朱晶, 劉帥, 賈維平, 等. 退火工藝對Ti+Nb-IF鋼組織與性能的影響. 熱加工工藝, 2008, 37(22):59 doi: 10.3969/j.issn.1001-3814.2008.22.019 [14] Petrovi? D S, Klan?nik G, Pirnat M, et al. Differential scanning calorimetry study of the solidification sequence of austenitic stainless steel. J Therm Anal Calorim, 2011, 105(1): 251 doi: 10.1007/s10973-011-1375-2 [15] Raju S, Ganesh B J, Banerjee A, et al. Characterisation of thermal stability and phase transformation energetics in tempered 9Cr–1Mo steel using drop and differential scanning calorimetry. Mater Sci Eng A, 2007, 465(1-2): 29 doi: 10.1016/j.msea.2007.01.127 [16] Wielgosz E, Kargul T. Differential scanning calorimetry study of peritectic steel grades. J Therm Anal Calorim, 2015, 119(3): 1547 doi: 10.1007/s10973-014-4302-5 [17] Ganjehfard K, Taghiabadi R, Noghani M T, et al. Tensile properties and hot tearing susceptibility of cast Al-Cu alloys containing excess Fe and Si. Int J Miner Metall Mater, 2021, 28(4): 718 doi: 10.1007/s12613-020-2039-7 [18] Ohnaka I. Mathematical analysis of solute redistribution during solidification with diffusion in solid phase. Trans Iron Steel Inst Jpn, 1986, 26(12): 1045 doi: 10.2355/isijinternational1966.26.1045 [19] Won Y M, Thomas B G. Simple model of microsegregation during solidification of steels. Metall Mater Trans A, 2001, 32(7): 1755 doi: 10.1007/s11661-001-0152-4 [20] Choudhary S K, Ghosh A. Mathematical model for prediction of composition of inclusions formed during solidification of liquid steel. ISIJ Int, 2009, 49(12): 1819 doi: 10.2355/isijinternational.49.1819 [21] You D L, Bernhard C, Wieser G, et al. Microsegregation model with local equilibrium partition coefficients during solidification of steels. Steel Res Int, 2016, 87(7): 840 doi: 10.1002/srin.201500216 [22] Ma Z T, Janke D. Characteristics of oxide precipitation and growth during solidification of deoxidized steel. ISIJ Int, 1998, 38(1): 46 doi: 10.2355/isijinternational.38.46 [23] Zhang X, Ma G J, Liu M K. Micro-segregation model calculation of residual tin in boiler and pressure vessel steel. Philos Mag, 2019, 99(9): 1041 doi: 10.1080/14786435.2019.1573333 -




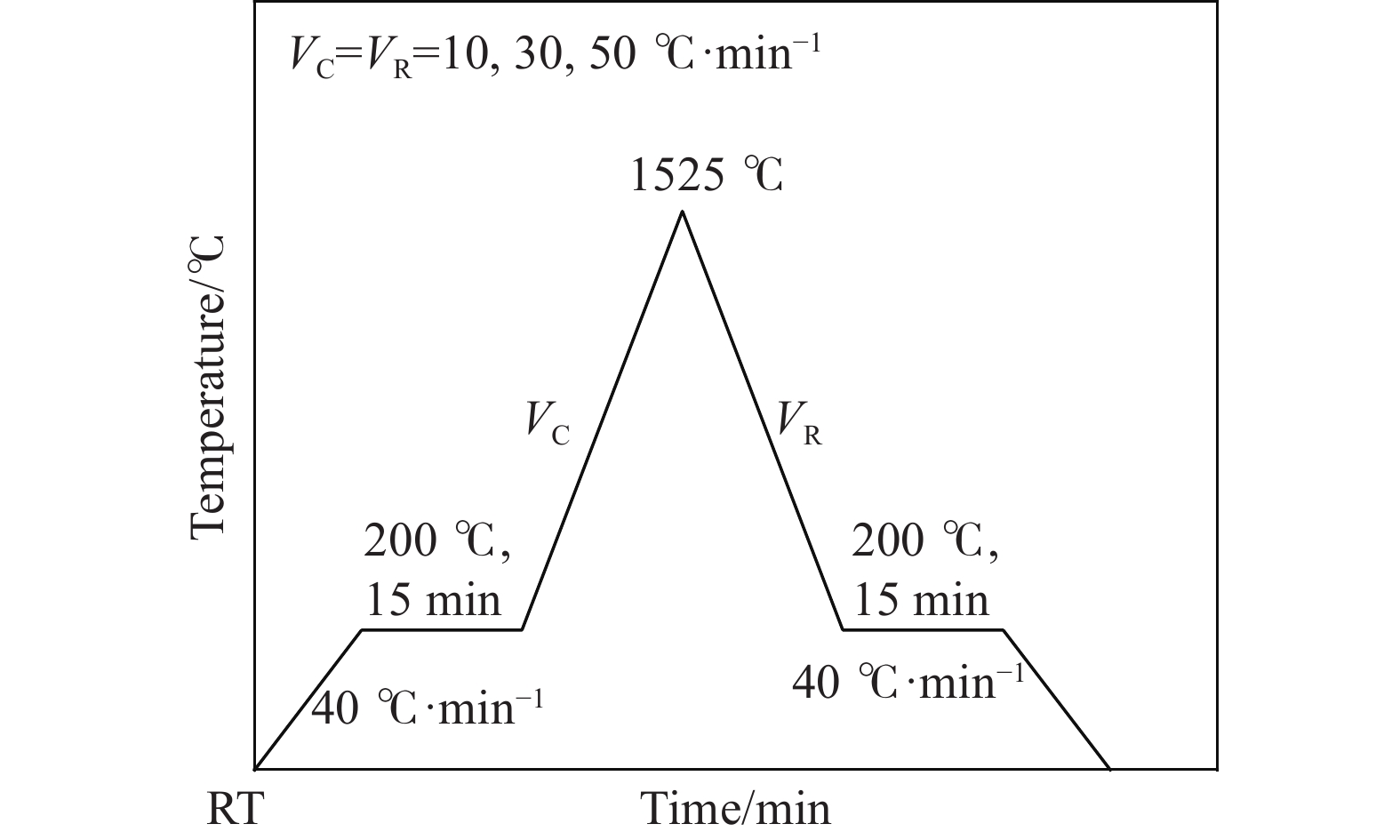
 下載:
下載: