-
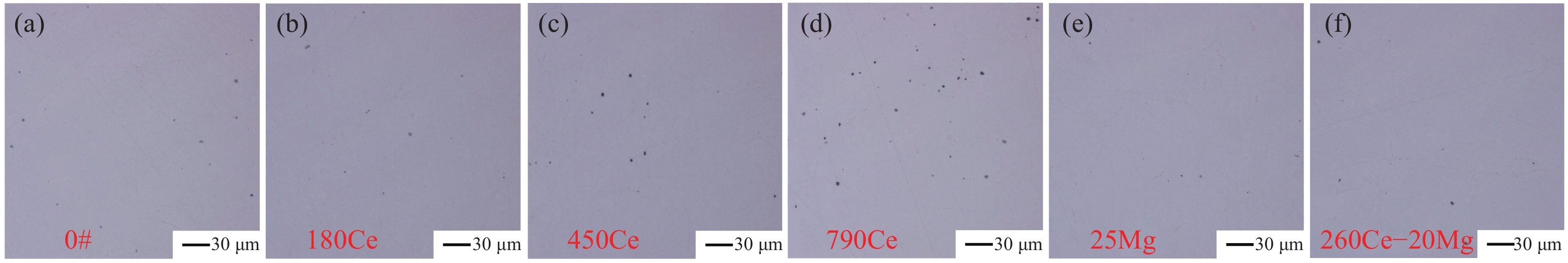
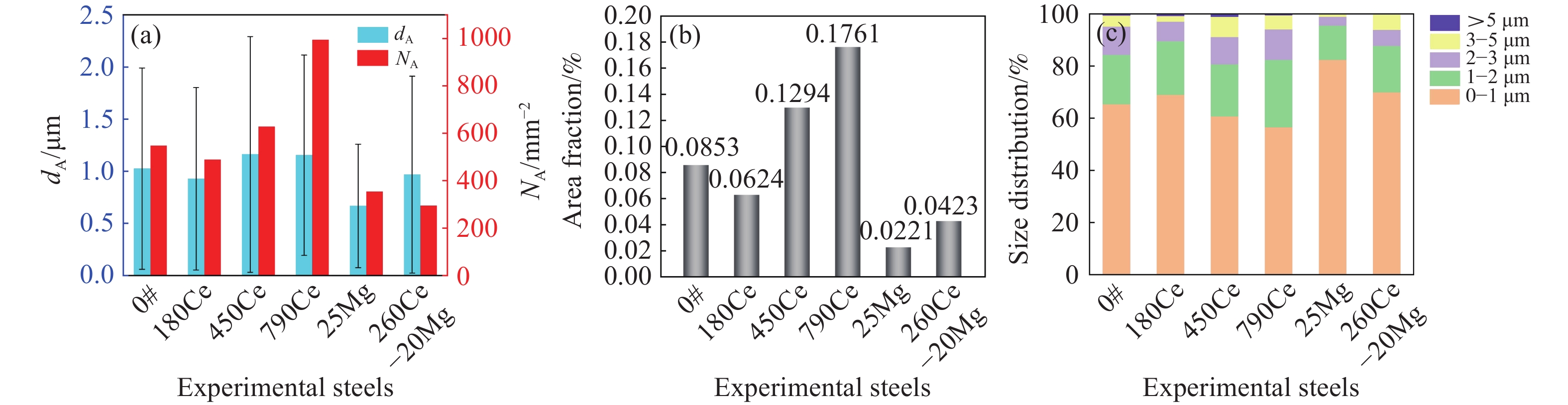
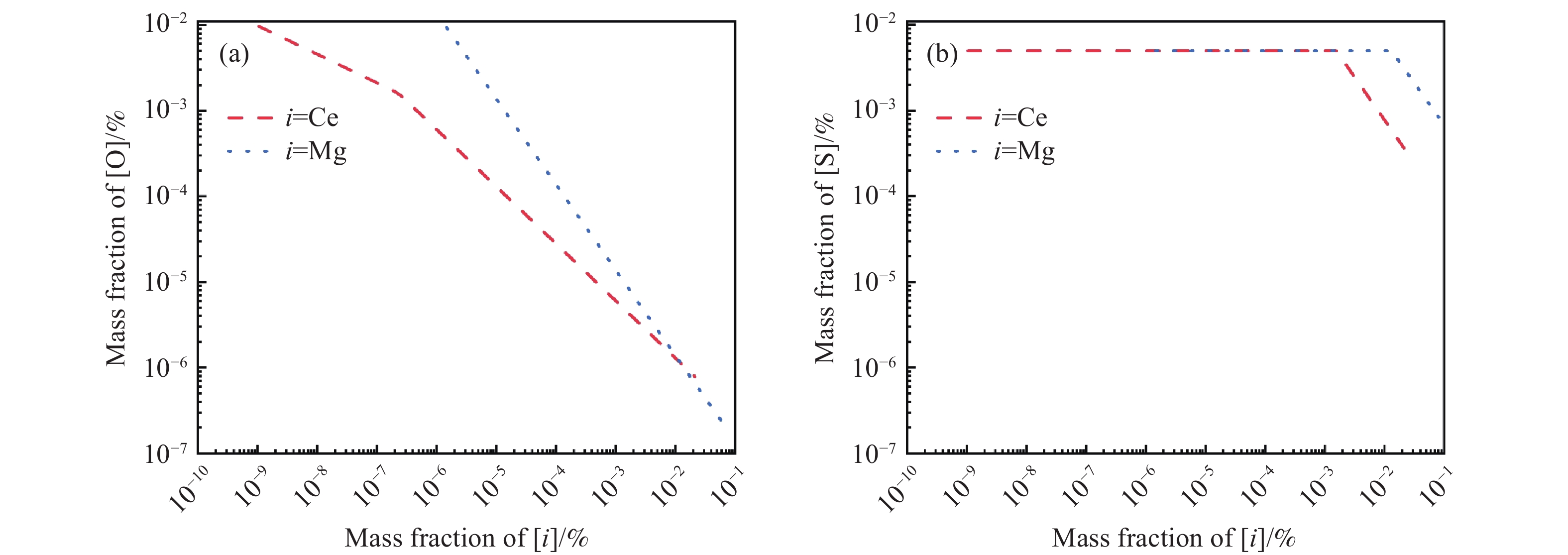
摘要: 采用真空感應熔煉工藝冶煉航空軸承鋼M50,對比分析了Ce處理、Mg處理和Ce–Mg復合處理對氧、硫含量和夾雜物分布特征的影響,結合熱力學計算,闡明了加入Ce、Mg元素對鋼液潔凈度的影響機理。研究發現,Ce具有很強的脫氧、脫硫能力,加入Ce會優先生成Ce2O2S夾雜物,隨著鋼液中氧含量的降低,Ce還會與As等有害雜質元素結合,起到凈化鋼液的效果。過量的Ce會加劇其與鎂鋁尖晶石材質耐火材料的反應,導致鋼中夾雜物數量的增加,Ce的質量分數為0.018%時,鋼中夾雜物的尺寸和數量最小;添加Mg不僅可以脫氧、脫硫,還可以抑制Ce與鎂鋁尖晶石耐材的反應,Ce–Mg復合處理可以顯著降低鋼中的夾雜物的尺寸和數量,將鋼中的氧的質量分數降低至0.00075%。Abstract: The cleanliness level and nonmetallic inclusion distribution characteristics of M50 aerospace-bearing steel are key factors affecting its quality and service life. The simultaneous addition of Ce–Mg has been proposed in this paper as an innovation to improve cleanliness dramatically. Based on the thermodynamic calculation, the underlying functional mechanism has been revealed. Additionally, the effects of Ce, Mg, and Ce–Mg simultaneous additions on oxygen content, sulfur content, and inclusion distribution characteristics have been analyzed comparatively. The vacuum induction melting process was used to prepare the M50 aerospace-bearing steel ingots. The chemical compositions of experimental steels were acquired using inductively coupled plasma-atomic spectroscopy, Leco TC500 N2/O2 analyzer, CS-3000 carbon/sulfur analyzer, and SPECTROLAB M11 stationary metal analyzer. The statistical distribution characteristics of inclusions were obtained using the image processing software based on optical microscopy images. The composition and morphology of inclusions have been characterized using scanning electron microscopy equipped with energy dispersive spectroscopy. The results indicated that Ce could significantly enhance the efficiency of deoxidation and desulfurization. Preferentially, Ce addition would lead to the formation of Ce2O2S inclusions in the steel. As the oxygen content in liquid steel decreases, Ce could also react with As to form a compound, and this could further purify the molten steel since As has generally been recognized as a harmful element. Meanwhile, Ce would also react with the magnesia–aluminum spinel refractory and cause an increase in the number density of inclusions in the steel. Thus, in comparison to the Ce-treated steel with higher Ce content, the smallest size and number of inclusions have been obtained in the steel with a total Ce mass fraction of 0.018%. In addition to deoxidation and desulfurization, Mg addition could also inhibit the reaction between Ce and magnesia–aluminum spinel refractories. The thermodynamic calculation results demonstrated that the dissolved [Ce] in the molten steel could react with the magnesia–aluminum spinel refractory material, resulting in an increase in the concentration of [O] and [Al] in the molten steel, while this reaction could significantly be inhibited by the dissolved [Mg] in the molten steel. In summary, Ce–Mg synergistic treatment could significantly decrease the number and size of inclusions in the steel. Based on this novel technology, the ultraclean M50 aerospace-bearing steel with an oxygen mass fraction of 0.00075% has successfully been obtained. This work has opened a new insight into the deoxidation mechanism of Ce–Mg synergistic treatment and provided a novel method to further improve the cleanliness of molten steel during the vacuum induction melting process.
-
Key words:
- Ce–Mg synergistic treatment /
- aerospace-bearing steel /
- inclusion /
- cleanliness /
- thermodynamics /
- refractory
-
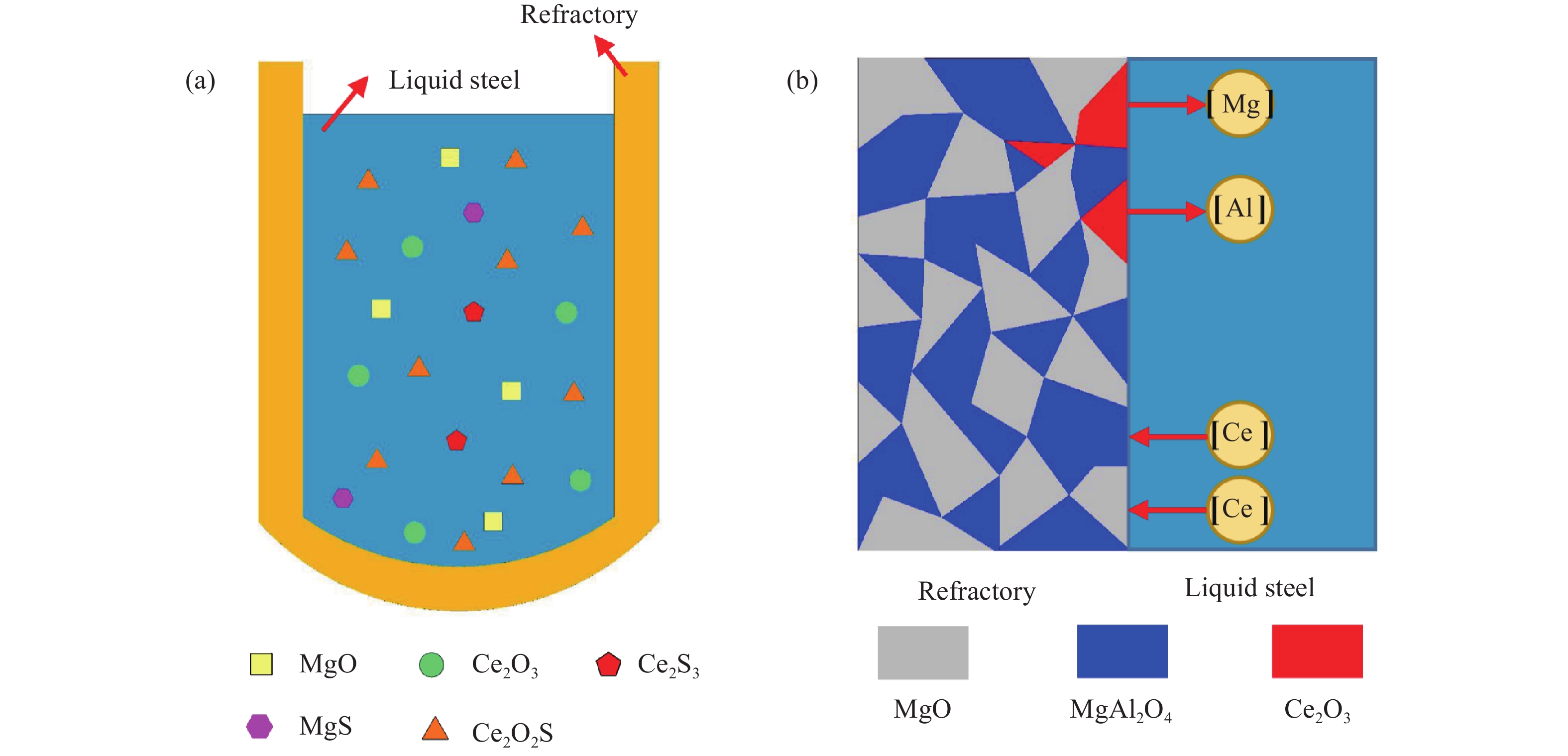
圖 7 Ce/Mg處理影響鋼液潔凈度的示意圖.(a) Ce/Mg處理生成的主要夾雜物;(b) 鋼液與耐材反應過程中的溶質擴散方向
Figure 7. Schematics showing the effect of Ce/Mg addition on the cleanliness of molten steel: (a) main inclusion types after Ce/Mg treatment; (b) diffusion direction of solutes in molten steel during the reaction between liquid steel and refractory materials
表 1 實驗鋼的化學成分(質量分數)
Table 1. Chemical compositions of experimental steels
% Steel C Mn Cr Mo V Ce Mg S Al O 0# 0.829 0.253 4.22 4.11 0.933 — — 0.0021 <0.010 0.00200 180Ce 0.825 0.258 4.23 4.11 0.939 0.018 — 0.0018 0.010 0.00152 450Ce 0.847 0.258 4.22 4.14 0.933 0.045 — 0.0017 0.013 0.00126 790Ce 0.832 0.256 4.24 4.14 0.945 0.079 — 0.0016 0.015 0.00114 25Mg 0.832 0.255 4.21 4.07 0.933 — 0.0025 0.0020 0.013 0.00147 260Ce–20Mg 0.839 0.252 4.24 4.10 0.932 0.026 0.0020 $\le$0.001 0.012 0.00075 表 2 實驗鋼中直徑大于1 μm的夾雜物類型
Table 2. Types of inclusions larger than 1 μm in diameter in experimental steels
Types of inclusions 0# 180Ce 450Ce 790Ce 25Mg 260Ce–20Mg SiO2 √√ √ √ — — √ Al2O3 √√ — √ √ — √ MnS √√ — — — — — MgS — — √ — √√ — Ce–S — √√ √√ √√ √ √√ Ce–O–(S) — √√ √√ √√ — √√ Mg–O–(S) — — — √ √√ √ Ca–O–(S) √ √ √ √ — √ xSiO2–yAl2O3 √ — √ √ — √ xAl2O3–yMnS √ — — — — — Mg–Al–O–(S) √ — — — √ — Ca–Mg–O–(S) √ — — — — √ Mg–Si–O– √ — √ — — — Ce–Al–O– — — √ √ — — Ce–Mg–O–(S) — — √ √ — — Ce–Mg–S — — √ — — √ Mg–Mn–S–(O) — — — — √ — Ca–Si–Al–O–(S) √ √ √ — — — Mg–Si–Al–O–(S) √ — — — — √ Ca–Mg–Si–Al–O–(S) √ — √ — — — Ce–Mg–Al–O — — — — — √ Note: √√—main type of inclusions; √—a small number of inclusions. 表 3 [Ce]、[Mg]與MgO、MgAl2O4之間發生化學反應的標準吉布斯自由能
Table 3. Standard Gibbs free energy for the chemical reactions between [Ce], [Mg] and MgO, MgAl2O4
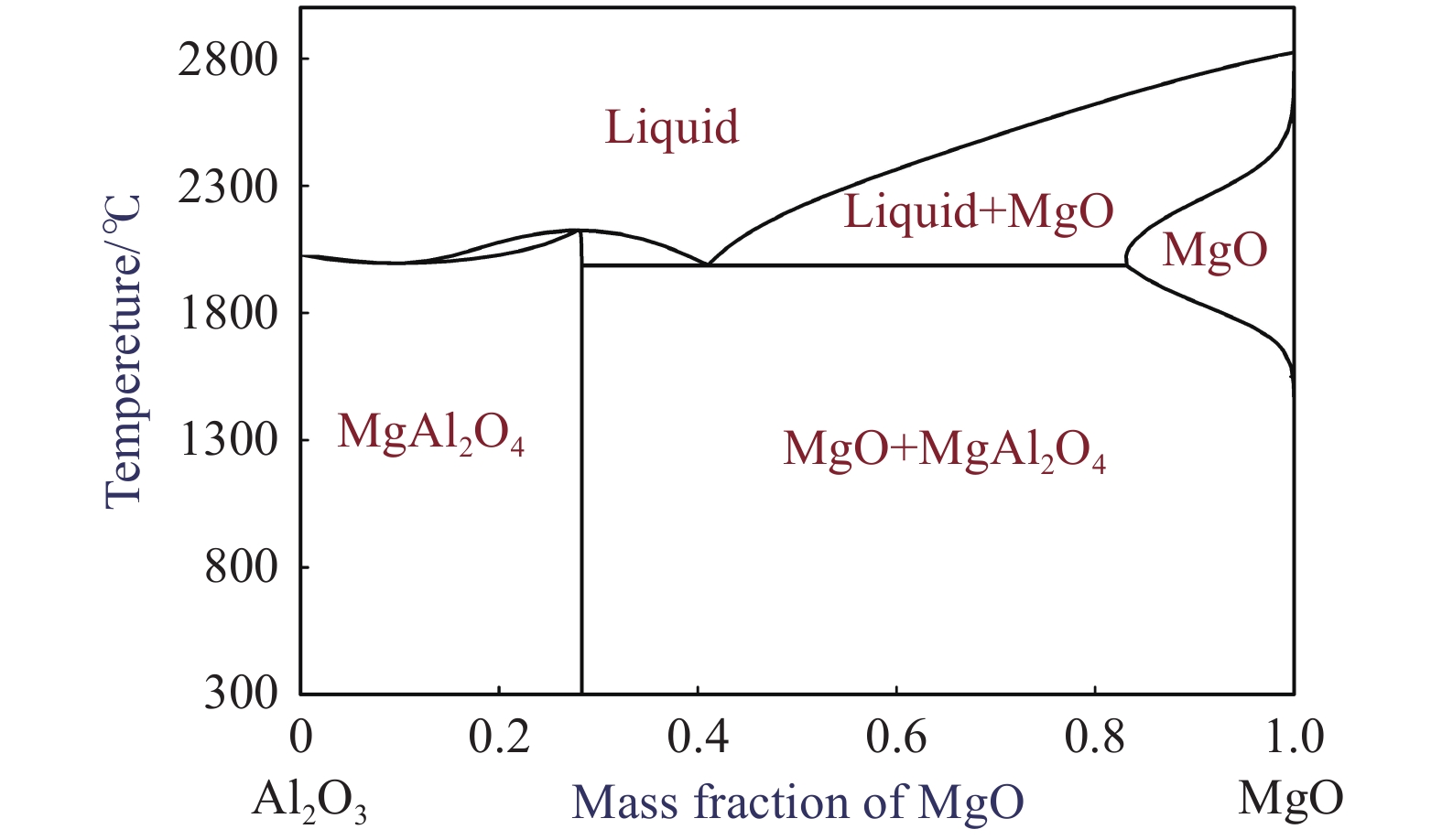
Equation No. Chemical reaction equations $\Delta {G^\ominus} $ / (J?mol–1) (1) [Ce] + 3/2MgO (s) = 1/2Ce2O3 (s) + 3/2[Mg] $\Delta {G^\ominus_1}$ = 382720 – 179.78T (2) [Ce] + 1/2[S] + MgO (s) = 1/2Ce2O2S (s) + [Mg] $\Delta {G^\ominus_2} $ = 55700 – 74.18T (3) [Ce] + 2MgO (s) = CeO2 (s) + 2[Mg] $\Delta {G^\ominus_3} $ = 610080 – 229.4T (4) [Ce] + 1/2MgAl2O4 (s) = 1/2Ce2O3 (s) + [Al]+ 1/2MgO (s) $\Delta {G^\ominus_4} $ = –270360 + 74.275T (5) [Ce] + 1/2[S] + 1/3MgAl2O4 (s) = 1/2Ce2O2S (s) + 2/3[Al]+ 1/3MgO (s) $\Delta {G^\ominus_5} $ = –79687 + 95.19T (6) [Ce] + 2/3MgAl2O4 (s) = CeO2 (s) + 4/3[Al]+ 2/3MgO (s) $\Delta {G^\ominus_6} $ = –260693 + 109.34T (7) [Ce] + 3/8MgAl2O4 (s) = 1/2Ce2O3 (s) + 3/4[Al]+ 3/8[Mg] $\Delta {G^\ominus_7} $ = –107090 + 10.76T (8) [Ce] + 1/2[S] + 1/4MgAl2O4 (s) = 1/2Ce2O2S (s) + 1/2[Al]+ 1/4[Mg] $\Delta {G^\ominus_8} $ = –270840 + 52.85T (9) [Ce] + 1/2MgAl2O4 (s) = CeO2 (s) + [Al]+ 1/2[Mg] $\Delta {G^\ominus_9} $ = –43000 + 24.655T (10) [Mg] + 1/3MgAl2O4 (s) = 4/3MgO (s) + 2/3[Al] $\Delta {G^\ominus_{10}}$ = –435387 + 169.37T 表 4 溫度為1823 K時鋼液中各組元的活度相互作用系數
$e^j_i$ Table 4. Activity interaction coefficients between various components in liquid steel at 1823 K
ji C Mn Cr Mo V Ce Mg S Al O Al 0.0085 0.0033 0.0140 0.0032 0.0094 –0.0023 0.0090 0.0306[15] 0.0816 –6.8289[17] Mn –0.0442 –0.0004 0.0029 –– 0.0004 –0.0008 –0.0091 –0.0445 0.0067 –0.0749[17] Ce –0.0799[17] –0.0017 0.0112 –– 0.0235 –0.0031[24] –0.4370 –8.6746[25] –0.0138 –5.2193[17] Mg 0.0208 –0.0041 0.0095 –– –0.0009 –0.0743 –0.2150 –1.4319[26] 0.0091 –477.311[27] O –0.4669[17] –0.0218[17] –0.0415[17] 0.0036[17] –0.3113[17] –0.5915[17] –311.289[27] –0.1380[17] –4.0468[17] –0.2075[17] S 0.1141[17] –0.0260 –0.0113 0.0028[17] –0.0166[17] –1.9819[25] –1.8885[28] –0.0291[17] 0.0363[17] –0.2802[17] 表 5 溫度為1823 K時[Ce], [Mg]與耐材之間化學反應的吉布斯自由能變化量
Table 5. Change in Gibbs free energy for the reaction between refractory material and [Ce] or [Mg] at 1823 K
J?mol–1 ΔG 0# 180Ce 450Ce 790Ce 25Mg 260Ce–20Mg ΔG1 –322933 –913481 –920953 –926723 548777 –46606 ΔG2 –63997 –656725 –665023 –670079 517561 –76007 ΔG3 –533374 –1119939 –1125271 –1130085 628931 37856 ΔG4 462006 –140371 –150216 –156644 466036 –143298 ΔG5 436911 –163663 –172193 –176952 441383 –161828 ΔG6 513212 –89125 –97621 –103313 518610 –91066 ΔG7 265769 –333651 –342902 –349166 486719 –119127 ΔG8 328477 –270165 –279649 –285035 476195 –124349 ΔG9 251565 –346829 –354533 –360006 546190 –58836 ΔG10 500907 493061 492829 493127 –76178 –85821 www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Li Z K, Lei J Z, Xu H F, et al. Current status and development trend of bearing steel in China and abroad. J Iron Steel Res, 2016, 28(3): 1 doi: 10.13228/j.boyuan.issn1001-0963.20150345李昭昆, 雷建中, 徐海峰, 等. 國內外軸承鋼的現狀與發展趨勢. 鋼鐵研究學報, 2016, 28(3):1 doi: 10.13228/j.boyuan.issn1001-0963.20150345 [2] Liu Y Z, Zhou L Y, Zhang C L, et al. Development and quality control of bearing steel for heavy equipment. Iron Steel, 2013, 48(8): 1劉雅政, 周樂育, 張朝磊, 等. 重大裝備用高品質軸承用鋼的發展及其質量控制. 鋼鐵, 2013, 48(8):1 [3] Jiang S F, Wang X L, Yuan Y T. Characteristics and application technology of precision machine tool bearings. Bearing, 2011(7): 57 doi: 10.3969/j.issn.1000-3762.2011.07.021姜韶峰, 王小龍, 袁玉同. 精密機床軸承的特點與應用技術. 軸承, 2011(7):57 doi: 10.3969/j.issn.1000-3762.2011.07.021 [4] Ye J. The localization of high-speed railway bearings is now dawning. Machinery, 2014, 52(6): 19葉軍. 高速鐵路軸承國產化現曙光. 機械制造, 2014, 52(6):19 [5] Li H W, Zhang B L, Chen L. Interview of academician Zhao Zhen-ye. Aeroengine, 2009, 35(3): 65李華文, 張寶玲, 陳磊. 趙振業院士訪談. 航空發動機, 2009, 35(3):65 [6] Yong Q L, Dong H, Liu Z D, et al. Recent progress in advanced machinery structure steels. Heat Treat Met, 2010, 35(1): 2 doi: 10.13251/j.issn.0254-6051.2010.01.025雍岐龍, 董瀚, 劉正東, 等. 先進機械制造用結構鋼的發展. 金屬熱處理, 2010, 35(1):2 doi: 10.13251/j.issn.0254-6051.2010.01.025 [7] Li X, Jiang Z H, Geng X, et al. Evolution mechanism of inclusions in H13 steel with rare earth magnesium alloy addition. ISIJ Int, 2019, 59(9): 1552 doi: 10.2355/isijinternational.ISIJINT-2019-094 [8] Liu Y Q, Wang L J, Chou K. Effect of cerium on the cleanliness of spring steel used in fastener of high-speed railway. J Rare Earths, 2014, 32(8): 759 doi: 10.1016/S1002-0721(14)60137-X [9] Kwon S K, Kong Y M, Park J H. Effect of Al deoxidation on the formation behavior of inclusions in Ce-added stainless steel melts. Met Mater Int, 2014, 20(5): 959 doi: 10.1007/s12540-014-5022-x [10] Karasev A, Suito H. Analysis of size distributions of primary oxide inclusions in Fe–10 mass Pct Ni-M (M=Si, Ti, Al, Zr, and Ce) alloy. Metall Mater Trans B, 1999, 30(2): 259 doi: 10.1007/s11663-999-0055-0 [11] Hong S H, Kang S J L, Yoon D N, et al. The reduction of the interfacial segregation of phosphorus and its embrittlement effect by lanthanum addition in a W-Ni-Fe heavy alloy. Metall Mater Trans A, 1991, 22(12): 2969 doi: 10.1007/BF02650256 [12] Wang H P, Xiong L, Zhang L, et al. Investigation of RE-O-S-As inclusions in high carbon steels. Metall Mater Trans B, 2017, 48(6): 2849 doi: 10.1007/s11663-017-1081-y [13] Takata R, Yang J, Kuwabara M. Characteristics of inclusions generated during Al-Mg complex deoxidation of molten steel. ISIJ Int, 2007, 47(10): 1379 doi: 10.2355/isijinternational.47.1379 [14] Kim H S, Chang C H, Lee H G. Evolution of inclusions and resultant microstructural change with Mg addition in Mn/Si/Ti deoxidized steels. Scr Mater, 2005, 53(11): 1253 doi: 10.1016/j.scriptamat.2005.08.001 [15] Yang C Y, Luan Y K, Li D Z, et al. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J Mater Sci Technol, 2019, 35(7): 1298 doi: 10.1016/j.jmst.2019.01.015 [16] Vahed A, Kay D A R. Thermodynamics of rare earths in steelmaking. Metall Mater Trans B, 1976, 7(3): 375 doi: 10.1007/BF02652708 [17] Chen J X. Data Manual of Common Steelmaking Charts. 2nd Ed. Beijing: Metallurgical Industry Press, 2010陳家祥. 煉鋼常用圖表數據手冊. 2版. 北京: 冶金工業出版社, 2010 [18] Liang Y J, Che Y C. Thermodynamic Data Handbook of Inorganic Materials. Shenyang: Northeast University Press, 1993梁英教, 車蔭昌. 無機物熱力學數據手冊. 沈陽: 東北大學出版社, 1993 [19] Liu D, Lei H, Wang T L, et al. Thermodynamics of deoxidation equilibrium with Al in liquid iron at 1873 K. J Mater Metall, 2015, 14(2): 96 doi: 10.14186/j.cnki.1671-6620.2015.02.005劉達, 雷洪, 王天龍, 等. 關于1873K下鐵液中鋁脫氧平衡熱力學的討論. 材料與冶金學報, 2015, 14(2):96 doi: 10.14186/j.cnki.1671-6620.2015.02.005 [20] Itoh H, Hino M, Ban-Ya S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall Mater Trans B, 1997, 28(5): 953 doi: 10.1007/s11663-997-0023-5 [21] Ueshima Y, Isobe K, Mizoguchi S, et al. Analysis of the rate of crystallization and precipitation of MnS in the resulphurized free-cutting steel. Tetsu-to-Hagane, 1988, 74(3): 465 doi: 10.2355/tetsutohagane1955.74.3_465 [22] Bale C W, Chartrand P, Degterov S A, et al. FactSage thermochemical software and databases. Calphad, 2002, 26(2): 189 doi: 10.1016/S0364-5916(02)00035-4 [23] Bale C W, Bélisle E, Chartrand P, et al. FactSage thermochemical software and databases, 2010-2016. Calphad, 2016, 54: 35 doi: 10.1016/j.calphad.2016.05.002 [24] Ma Q Q, Wu C C, Cheng G G, et al. Characteristic and formation mechanism of inclusions in 2205 duplex stainless steel containing rare earth elements. Mater Today Proc, 2015, 2: S300 doi: 10.1016/j.matpr.2015.05.042 [25] Li W C, Lin Q, Ye W, et al. Thermodynamical analysis of the formation of rare earth inclusions in 35CrNi3MoV steel. J Chin Rare Earth Soc, 1984, 2(2): 57 doi: 10.3321/j.issn:1000-4343.1984.02.009李文超, 林勤, 葉文, 等. 35CrNi3MoV鋼中稀土夾雜物生成的熱力學計算. 中國稀土學報, 1984, 2(2):57 doi: 10.3321/j.issn:1000-4343.1984.02.009 [26] Wu Z, Li J, Shi C B, et al. Effect of magnesium addition on inclusions in H13 die steel. Int J Miner Metall Mater, 2014, 21(11): 1062 doi: 10.1007/s12613-014-1010-x [27] Ohta H, Suito H. Deoxidation equilibria of calcium and magnesium in liquid iron. Metall Mater Trans B, 1997, 28(6): 1131 doi: 10.1007/s11663-997-0069-4 [28] Nadif M, Gatellier C. Influence of calcium and magnesium on the solubility of oxygen and sulphur in liquid steel. Rev Met Paris, 1986, 83(5): 377 doi: 10.1051/metal/198683050377 -




 下載:
下載: