-
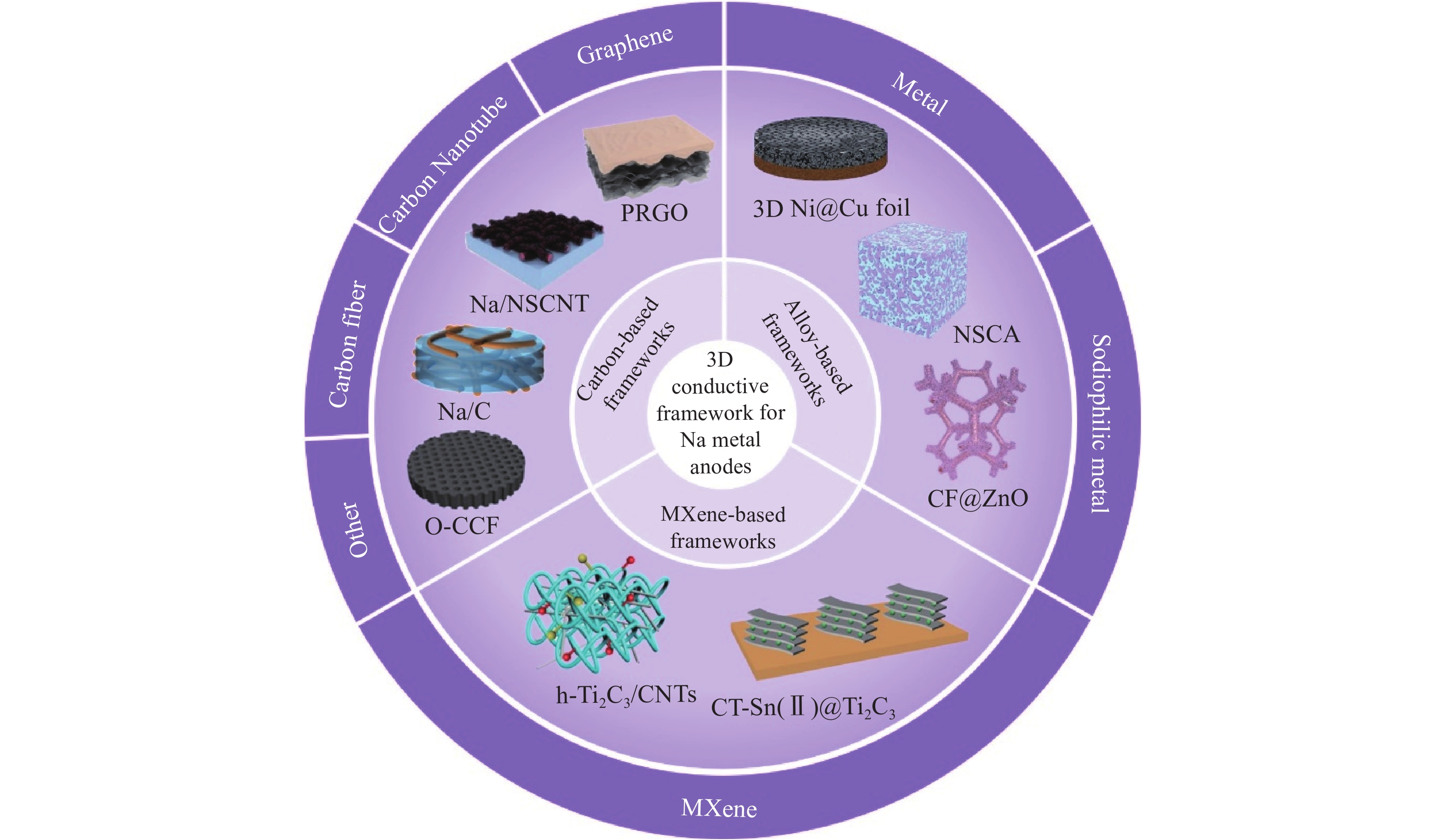
摘要: 鈉金屬因其成本低、自然豐度高、氧化還原電位低和理論比容量高等優點,被認為是高能電池的理想負極材料。然而,鈉金屬在充放電過程中易發生體積膨脹和產生鈉枝晶,導致電池性能不斷惡化,并引發安全隱患,嚴重阻礙了鈉金屬電池在實際中的應用。為了解決上述問題,國內外已進行了大量探索。其中,構建三維導電載體可以有效降低局部電流密度和成核能,抑制枝晶生長和減緩體積膨脹,在未來應用方面具有巨大的潛力。本文綜述了近年來利用三維導電載體來提高鈉金屬負極電化學循環穩定性的研究進展并對三維導電載體進行了總結和分類。最后,從基礎研究和實際應用兩個方面討論了三維導電載體材料在鈉金屬負極中的發展前景和未來研究方向。Abstract: Sodium is considered an ideal anode material for high-energy batteries because of its low cost, high natural abundance, low redox potential (?2.71 V vs SHE), and high theoretical specific capacity (1166 mA·h·g?1). However, due to the high reactivity, sodium rapidly reacts with the electrolyte to form an unstable solid electrolyte interface (SEI) layer during stripping/plating cycling. In addition, due to the large size change of sodium, the SEI layer repeatedly breaks and reassembles, resulting in the continuous consumption of sodium and electrolyte, as well as low coulombic efficiency and rapid capacity loss. Simultaneously, due to an uneven electric field distribution on sodium, numerous sodium dendrites generate during the repeated plating/stripping cycles. The growing Na dendrites easily pierce the separator, causing a short circuit and a series of safety issues. The above issues lead to the deterioration of battery performance and safety risks, thus considerably hindering the application of sodium metal batteries. Various studies have been conducted to solve these issues, including electrolyte engineering, artificial SEI layers, current collector and interlayer engineering, solid-state electrolyte engineering, and three-dimensional (3D) frameworks for sodium metal. Among various improvement strategies, the construction of a 3D conductive framework can effectively reduce the local current density, decrease nuclear energy, inhibit Na dendrite growth, and impede volume expansion, thus having a great potential in future applications. In this study, the current research progress in using various 3D conductive frameworks to improve the cycling stability of a sodium metal battery is reviewed, including carbon-based, metal-based, and MXene-based frameworks. Simultaneously, the pros and cons of different 3D conductive framework technologies in recent years are summarized and classified, and the electrochemical performance parameters of different 3D conductive frameworks for sodium metal batteries are compared. Finally, the development prospect and direction of 3D conductive frameworks in sodium metal anodes are discussed from basic research and practical applications. This review provides deeper insights into building more comprehensive and efficient sodium metal anodes. The 3D conductive framework technology can remarkably improve the cycle life and safety of a sodium metal battery. Multistrategy joint research methods will facilitate the practical applications of a sodium metal battery. Further exploration of the deposition behavior of sodium metal is required in the future, and we believe that it can definitely achieve commercial applications with continuous efforts.
-
Key words:
- sodium metal battery /
- anode /
- Na dendrite /
- volume expansion /
- three-dimensional conductive framework
-
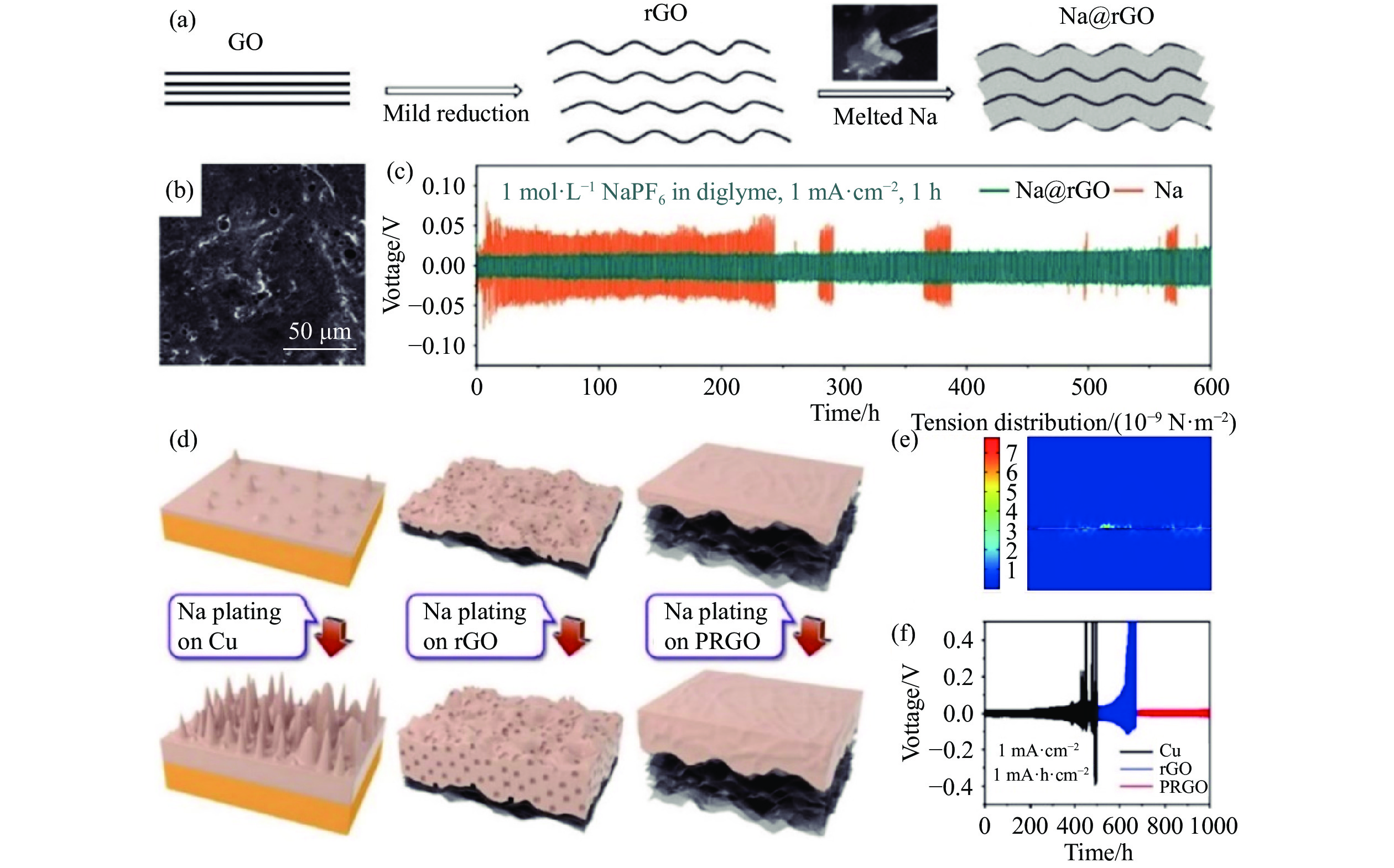
圖 1 (a) Na@rGO復合負極的合成過程示意圖[54]; (b) Na@rGO的表面SEM圖像[54]; (c) Na/Na和Na@rGO/Na@rGO 對稱電池的恒流充放電循環[54]; (d) Cu箔、rGO和PRGO薄膜上Na金屬的沉積示意圖[56]; (e) Na金屬在PRGO薄膜上沉積的力學模擬[56]; (f) 在1 mA·cm?2、1 mA·h·cm?2條件下,三種不同載體對稱電池的恒流充放電循環[56]
Figure 1. (a) Schematic of the preparation of Na@rGO composites[54]; (b) top-view SEM images of Na@rGO[54]; (c) galvanostatic cycling of symmetric Na/Na and Na@rGO/Na@rGO cells after 300 cycles[54]; (d) schematic of Na nucleation and growth on Cu foil, planar rGO film, and flexible PRGO film, respectively[56]; (e) tension schematics for Na plating on PRGO films through mechanical simulation[56]; (f) symmetric cell patterns of Na plating on three matrices with the capacity limitation of 1 mA·h·cm?2 at the current density of 1 mA·cm?2[56]
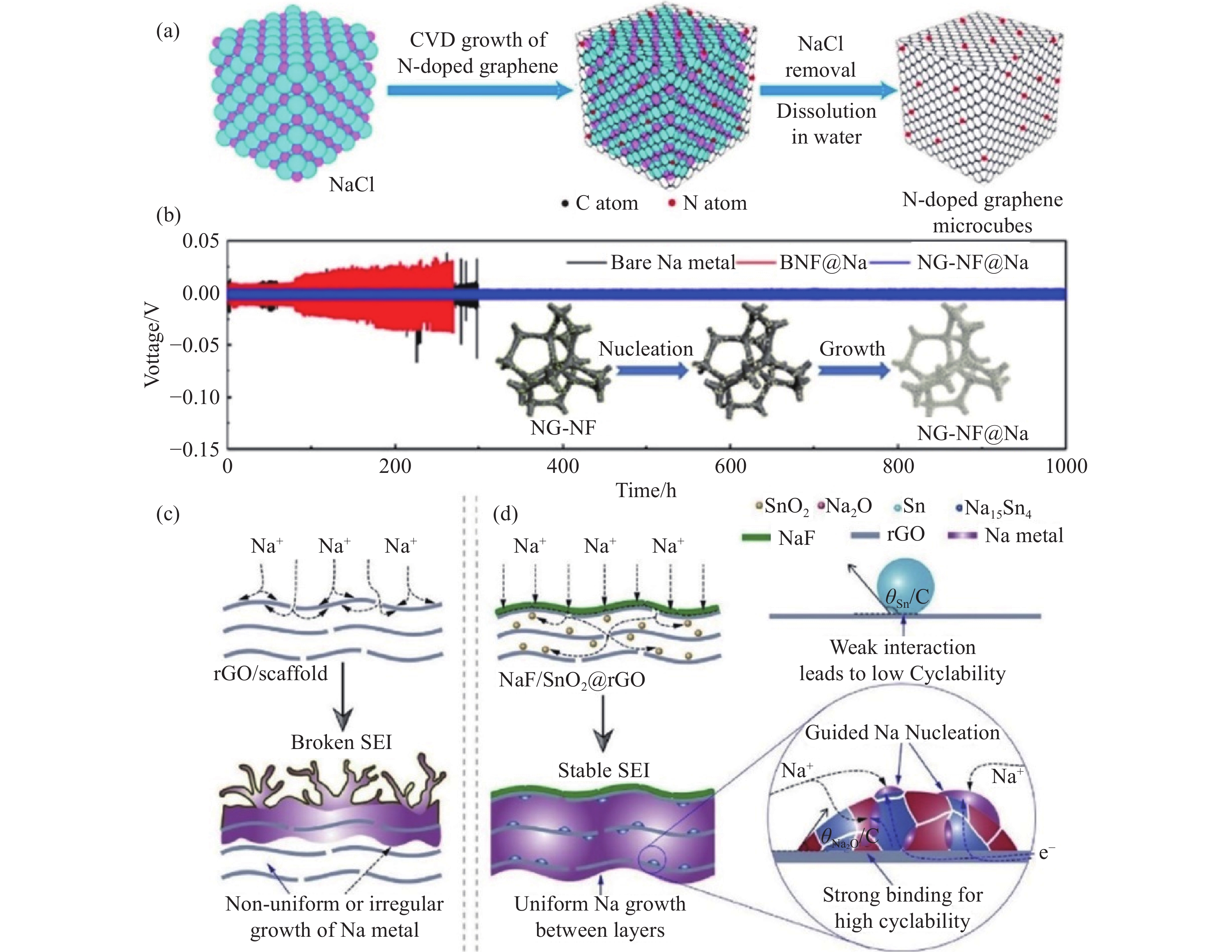
圖 2 (a) 氮摻雜石墨烯立方體 (PN-G) 復合結構的制備示意圖[58]; (b) 鈉在NG-NF電極上的成核和生長過程,以及在1 mA·cm?2、1 mA·h·cm?2條件下,Na、BNF@Na和NGNF@Na對稱電池的恒電流充放電曲線[59]; 鈉金屬在 (c) rGO載體和 (d) NaF/SnO2@rGO載體的沉積示意圖[60]
Figure 2. (a) Schematic of N-doped graphene microcube (PN-G) preparation[58]; (b) schematic of the Na nucleation and growth process on the NG-NF electrode and voltage profiles of Na plating/stripping in three symmetric cells (Na foil, BNF@Na, and NGNF@Na cells) at 1 mA·cm?2 for 1 mA·h·cm?2 [59]; schematic of the Na deposition process: (c) nonuniform or irregular growth of Na metal on rGO or scaffolds; (d) guided uniform Na plating in NaF/SnO2@rGO[60]
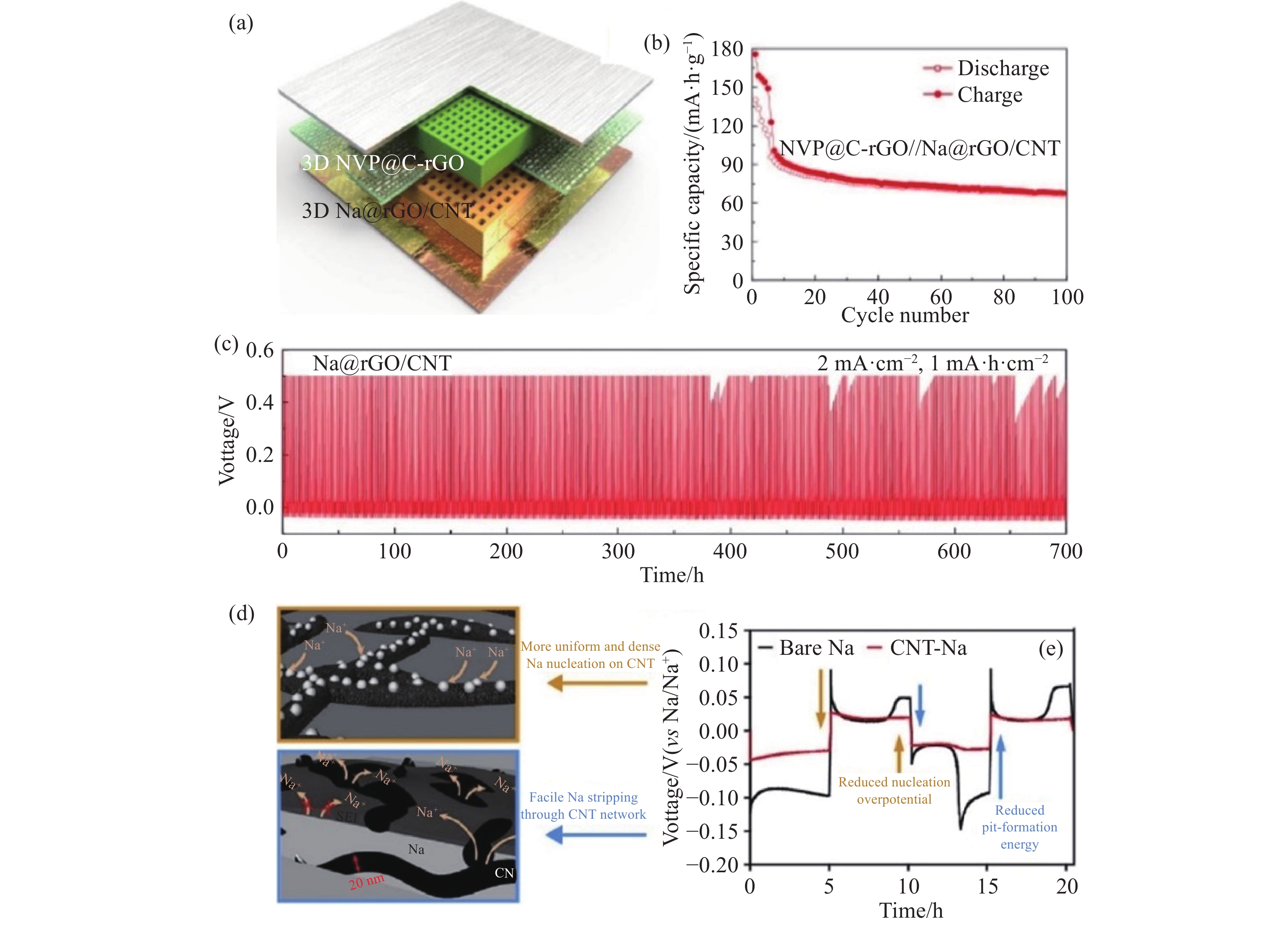
圖 3 (a) 通過3D打印制備的NVP@C-rGO正極/Na@rGO/CNT負極的全電池示意圖[61]; (b) 全電池在電流密度為電流密度為100 mA·g?1時的循環性能[61]; (c) Na@rGO/CNT電極在電流密度為2 mA·cm?2、容量為1 mA·h·cm?2時的恒電充放曲線上[62]; (d)裸鈉和CNT-Na復合電極上的初始鈉成核示意[62]; (e)裸鈉和CNT-Na對稱電池的恒電流循環曲線 (1 mA·h·cm?2, 0.5 mA·cm?2)[62]
Figure 3. (a) Schematic of the 3D-printed microlattice sodium ion full batteries with NVP@C-rGO as the cathode and Na@rGO/CNT as the anode[61]; (b) cycling performance at a current density of 100 mA·g?1[61]; (c) cycling performance of the Na@rGO/CNT electrodes at a high current density of 2 mA·cm?2 with a capacity limitation of 1 mA·h·cm?2[62]; (d) schematic of initial Na nucleation on bare Na and CNT-Na composite electrodes; (e) galvanostatic cycling profiles of the Na/Na symmetric cells with bare Na and CNT-Na electrodes (1 mA·h·cm?2, 0.5 mA·cm?2) [62]
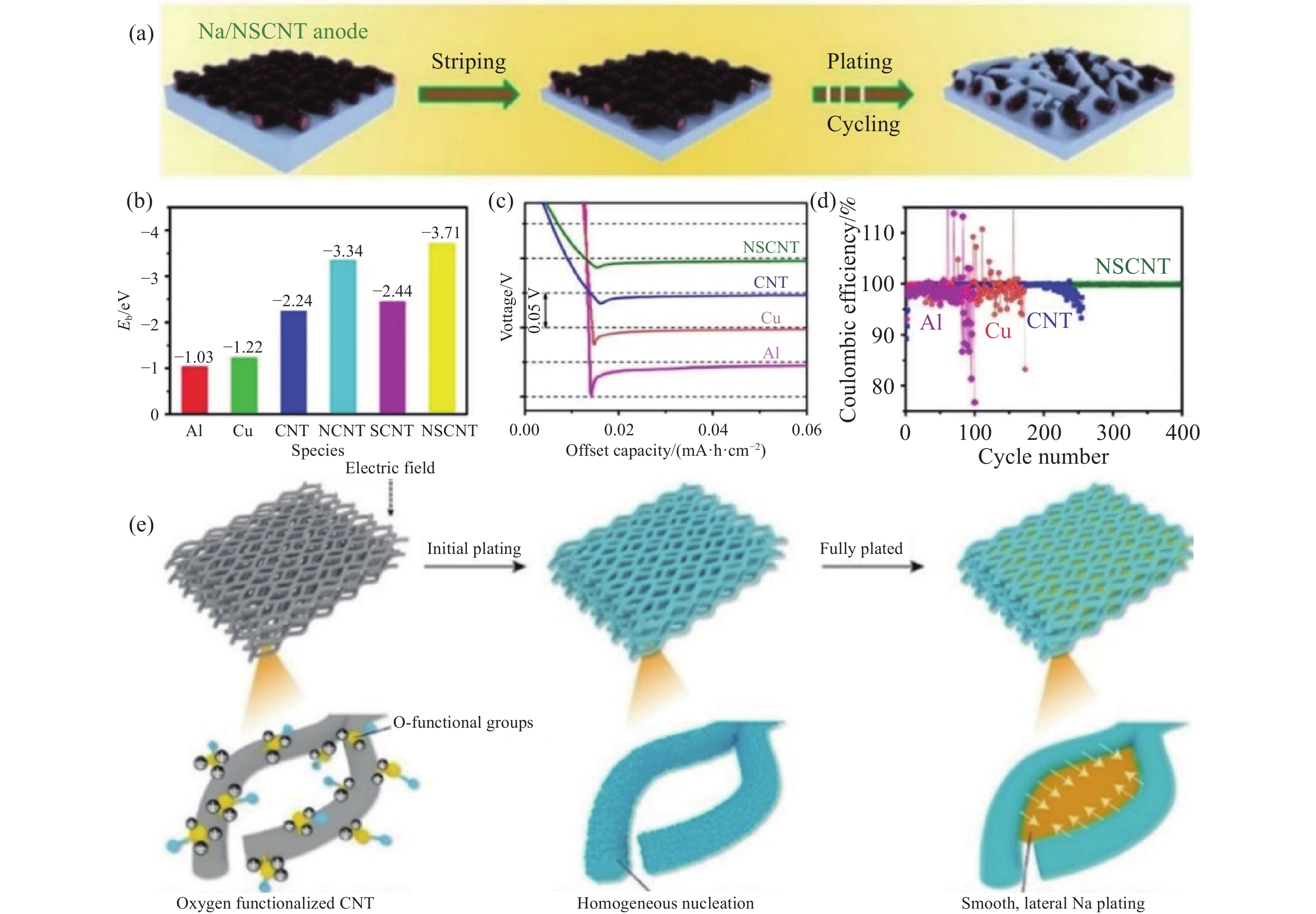
圖 4 (a) 鈉金屬在Na/NSCNT負極上的沉積示意圖[63]; (b) 鈉與Al、Cu、CNT、NCNT、SCNT、NSCNT的結合能(Eb)[63]; (c) 電流密度為0.05 mA·cm?2時,不同集流體的初始成核能[63]; (d) 在電流密度為1 mA·cm?2、沉積容量為1 mA·h·cm?2時,Cu、Al、CNT和NSCNT的庫侖效率[63]; (e) 在Of?CNT載體中,鈉金屬均勻沉積示意圖[64]
Figure 4. (a) Schematic of the Na striping/plating on the Na/NSCNT anode[63]; (b) binding energies of Na atoms with Al, Cu, CNT, NCNT, SCNT, and NSCNT[63]; (c) the potential-capacity profiles during Na nucleation on different current collectors at a current density of 0.05 mA·cm?2[63]; (d) coulombic efficiencies of Na plating/stripping on Cu foil, Al foil, CNT paper, and NSCNT paper at a current density of 1 mA·cm?2 with a capacity of 1 mA·h·cm?2 [63]; (e) schematic of the Na striping/plating on Of?CNT skeleton[64]
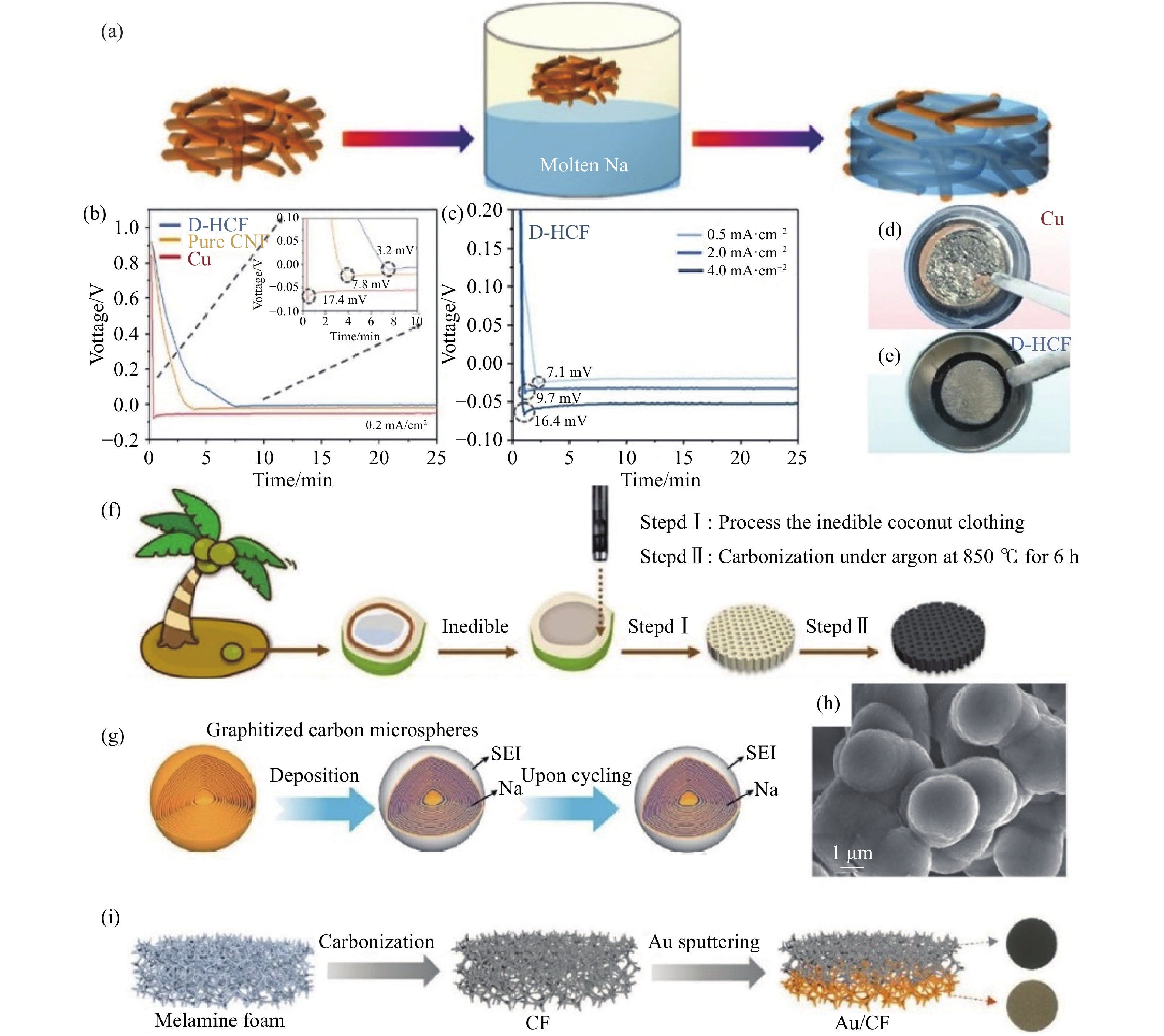
圖 5 (a) 鈉-碳氈 (Na/C)復合電極的制備[67]; (b) 鈉金屬在Cu、純泡沫鎳 (CNF) 和D-HCF電極上的初始成核能[71]; (c) 不同電流密度下D-HCF的初始成核能[71]; (d,e) 8.0 mA·h·cm?2的鈉金屬沉積在Cu箔和D-HCF上的光學照片[71];(f) 利用生物質廢棄椰衣制備3D O-CCF載體的示意圖[19]; (g) 金屬鈉封裝在碳納米薄片中的示意圖[76]; (h) 鈉金屬沉積后石墨化碳微球的SEM圖像[76]; (i) Au/CF載體的制備示意圖[77]
Figure 5. (a) Fabrication of the Na/C composite anode[67]; (b) the initial voltage profiles on planar Cu, pure nickel foam (CNF), and D-HCF electrode[71]; (c) the initial voltage profiles on D-HCF at various current densities [71]; optical photos of Na deposition on (d) planar Cu and (e) D-HCF with an aerial loading of 8.0 mA·h·cm?2[71]; (f) fabrication schematic of 3D O-CCF matrix from biomass waste coconut coat[19]; (g) schematic of the encapsulated Na configuration where most nanoscale metallic Na is embedded inside the graphitized nanosheets[76]; (h) SEM image of the GCMs after Na deposition[76]; (i) fabrication schematic of the Au/CF host[77]
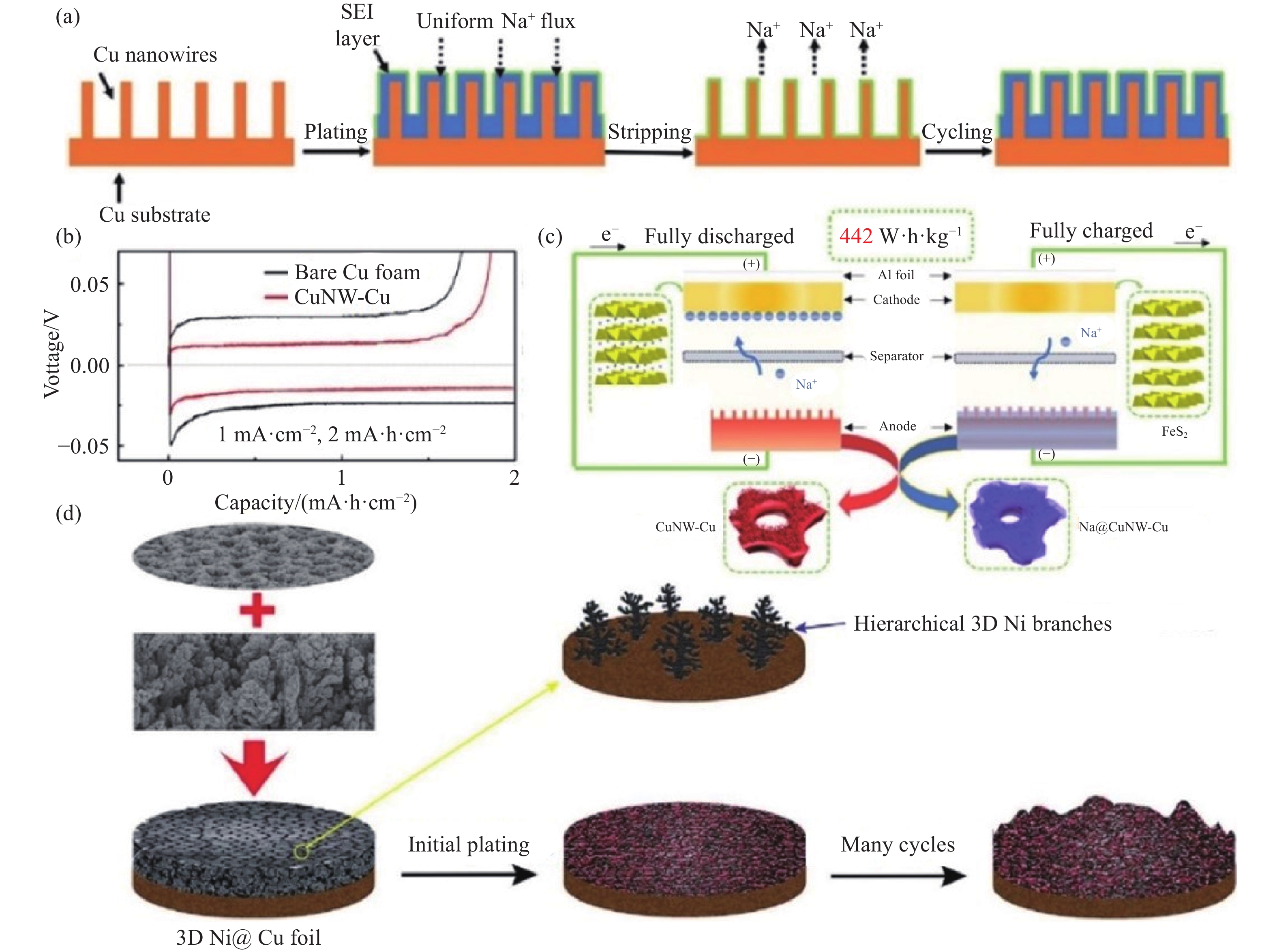
圖 6 (a)鈉金屬在CuNW-Cu上循環沉積示意圖[80]; (b) 裸泡沫銅和CuNW-Cu的首次充放電曲線[80]; (c)全電池充放電示意圖[80]; (d) 3D Ni@Cu中鈉金屬的沉積示意圖[79]
Figure 6. (a) Schematic of the Na plating processes on the CuNW-Cu substrate[80]; (b) first charge?discharge profiles of bare Cu foam and CuNW-Cu[80]; (c) illustration of completely charged and discharged states of the full cells[80]; (d) schematic of the Na plating process on 3D Ni@Cu[79]
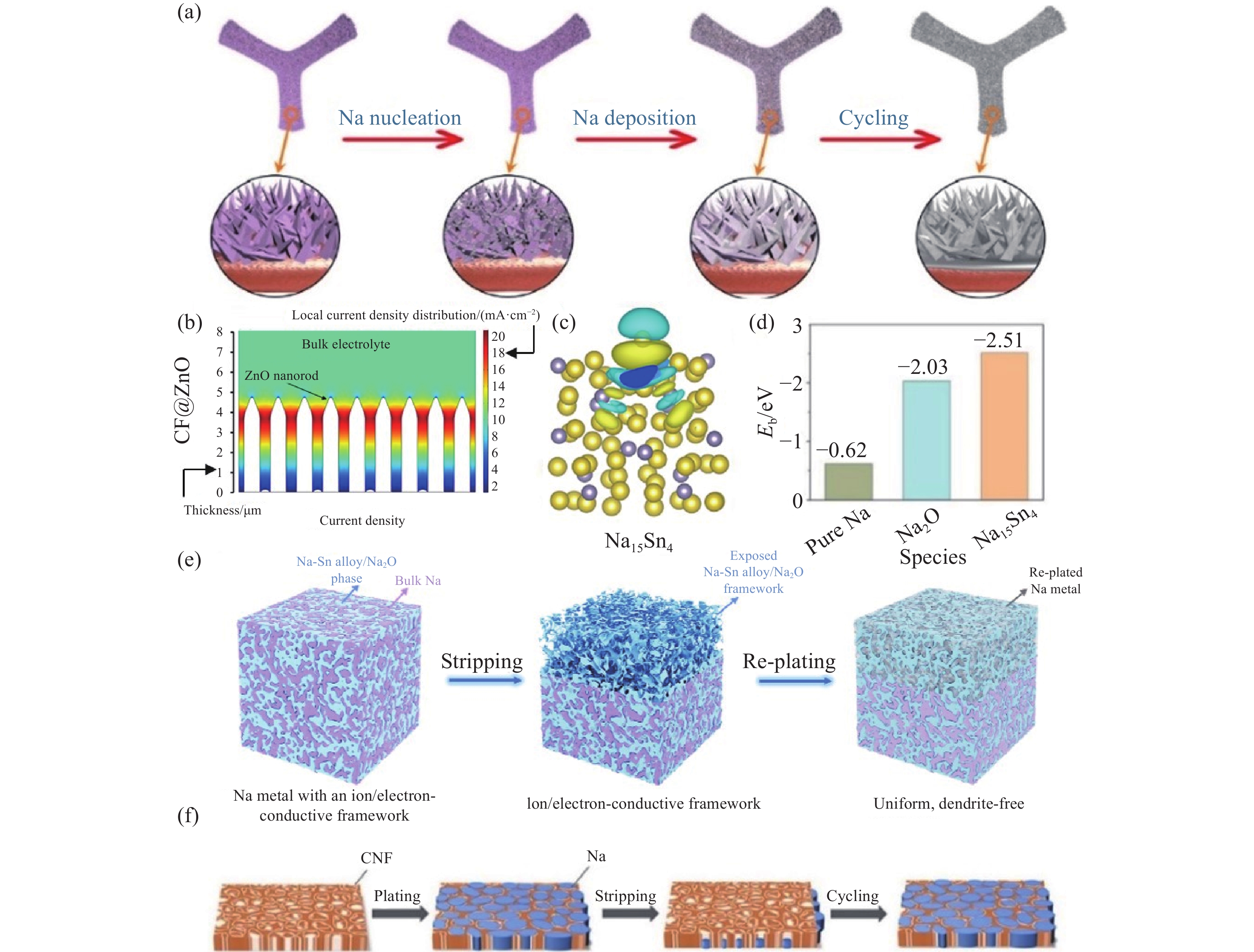
圖 7 (a) 鈉金屬在CF@ZnO上的成核和沉積過程[82]; (b) CF@ZnO界面局部電流密度分布的多物理場仿真模擬[82]; (c) 鈉在Na15Sn4上的電荷密度[82]; (d) 鈉在純Na、Na2O和Na15Sn4上的結合能[83]; (e) 鈉金屬在Na-Sn合金/Na2O載體上的沉積/溶解示意圖[83]; (f) Na在CNF上的沉積示意圖[75]
Figure 7. (a) Schematic of the Na nucleation and deposition processes on CF@ZnO[82]; (b) COMSOL simulation of the local current density distribution at the substrate/electrolyte interface of CF@ZnO[82]; (c) charge density for Na on Na15Sn4[82]; (d) bar chart on the summary of the calculated binding energy of Na on pure Na, Na2O, and Na15Sn4[83]; (e) Na stripping and plating process on the Na–Sn alloy/Na2O framework[83]; (f) schematic of Na stripping/plating on CNF[75]
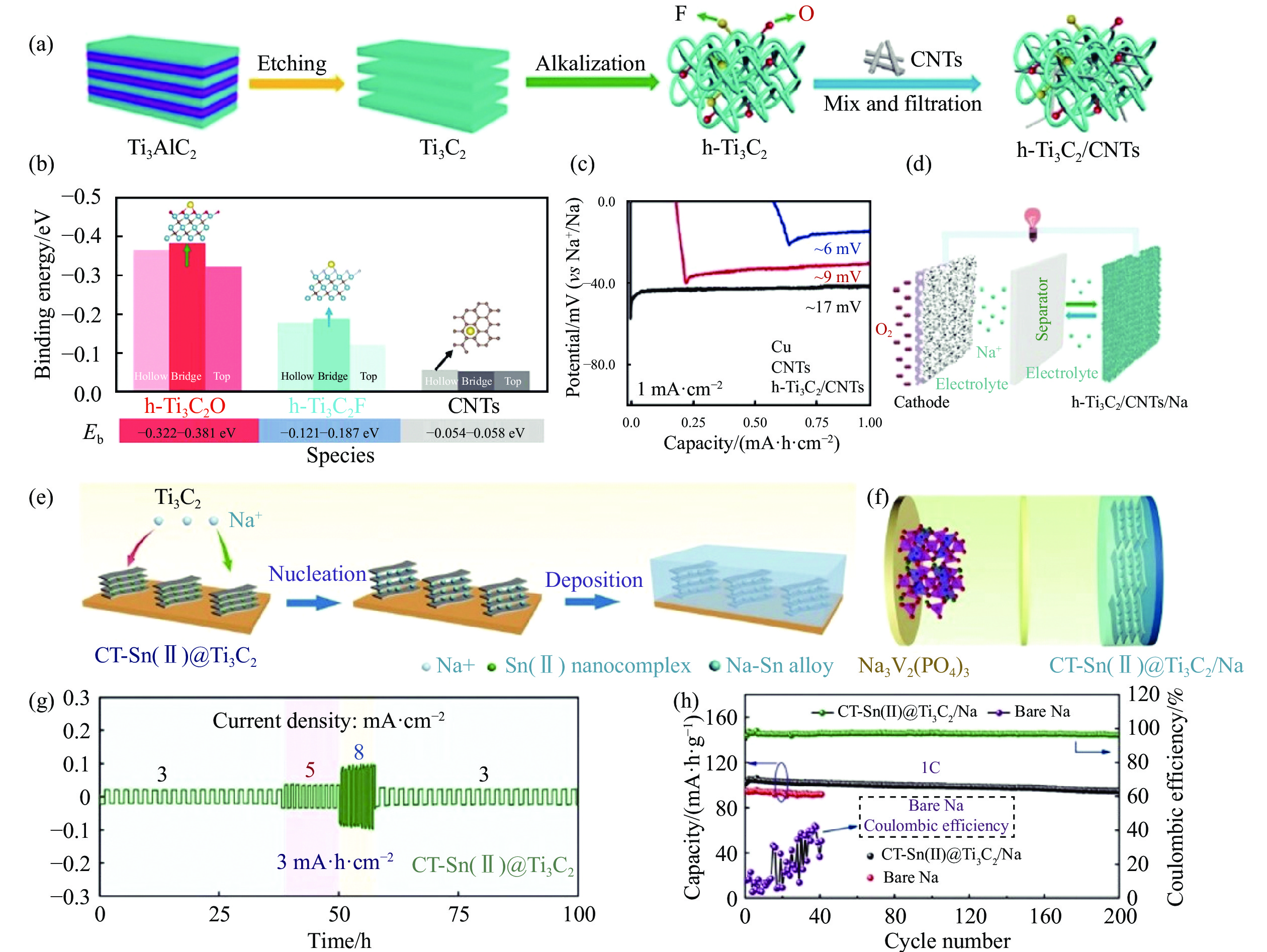
圖 8 (a) h-Ti3C2/CNTs的合成示意圖[84]; (b) 鈉原子與碳原子 (CNTs)、氧原子 (h-Ti3C2O) 和氟原子 (h-Ti3C2F)的結合能[84]; (c) 鈉在Cu、CNTs和h-Ti3C2/CNTs載體上的初始成核能[84]; (d) Na//O2電池示意圖[84]; (e) 鈉金屬在CT-Sn(II)@Ti3C2載體上的成核和沉積示意圖[86]; (f) Na3V2(PO4)3//CT-Sn(II)@ Ti3C2/Na全電池示意圖[86]; (g) CT-Sn(II)@Ti3C2對稱電池的倍率性能[86]; (h) Na3V2(PO4)3/Na和Na3V2(PO4)3/CT-Sn(II)@Ti3C2/Na全電池在1C條件下的循環性能[86]
Figure 8. (a) Synthesis of h-Ti3C2/CNTs[84]; (b) corresponding binding energies of a Na atom with C atom (CNTs), O atom (h-Ti3C2O), and F atom (h-Ti3C2F) [84]; (c) nucleation overpotentials for Na plating on Cu, CNTs, and h-Ti3C2/CNTs hosts (at 1 mA·cm?2) [84]; (d) graph of the Na//O2 battery[84]; (e) schematics for the comparison of Na nucleation and deposition in CT-Sn(II)@Ti3C2 matrixes[84]; (f) full-cell configurations of Na3V2(PO4)3//CT-Sn(II)@Ti3C2/Na cells[86]; (g) rate performance of the CT-Sn(II)@Ti3C2 symmetric cell[86]; (h) cycling performance of Na3V2(PO4)3//bare Na and Na3V2(PO4)3//CT-Sn(II)@Ti3C2/Na cells at 1C[86]
表 1 使用不同3D導電載體的鈉金屬電池(SMBs) 的電化學性能
Table 1. Electrochemical performance parameters of different 3D conductive frameworks for SMBs
Ref. Materials Electrolyte Current density/ Capacity/ Time/ Coulombic efficiency/% (mA·cm?2) (mA·h·cm?2) h 19 O-CCF NaPF6 in diglyme 5 5 4000 99.80 54 Na@rGO NaPF6 in diglyme 1 1 600 56 PRGO NaPF6 in EC/DMC 1 1 1000 99.50 58 PN-G NaClO4 in EC/PC 5 3 600 59 NG-NF NaPF6 in diglyme 0.5 1 800 >99 60 NaF/SiO2@rGO NaSO3CF3 in diglyme 1 0.5 3000 99.87 61 rGO/CNT NaPF6 in diglyme 1 1 800 99.50 62 CNT-Na NaClO4 in EC/DMC 0.5 1 700 63 NSCNT NaSO3CF3 in diglyme 1 1 500 99.80 64 Of-CNTs NaPF6 in diglyme 1 1 4000 67 Na/C NaClO4 in EC/PC 1 2 27000 71 D-HCF NaSO3CF3 in diglyme 0.5 1 700 77 Au/CF NaCF3SO3 in diglyme 2 1 1000 99.50 80 CuNW-Cu NaPF6 in diglyme 1 2 1400 97.50 82 CF@ZnO NaPF6 in diglyme 1 3 500 99.50 83 Na-Sn alloy/Na2O NaClO4 in EC/PC 1 1 160 84 h-Ti3C2/CNTs NaCF3SO3 in diglyme 1 1 4000 99.20 86 CT-Sn(II)@Ti3C2 NaPF6 in diglyme 4 4 500 83.70 www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Delmas C. Sodium and sodium-ion batteries: 50 years of research. Adv Energy Mater, 2018, 8(17): 1703137 doi: 10.1002/aenm.201703137 [2] Lee B, Paek E, Mitlin D, et al. Sodium metal anodes: Emerging solutions to dendrite growth. Chem Rev, 2019, 119(8): 5416 doi: 10.1021/acs.chemrev.8b00642 [3] Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: A battery of choices. Science, 2011, 334(6058): 928 doi: 10.1126/science.1212741 [4] Liu T F, Zhang Y P, Jiang Z G, et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ Sci, 2019, 12(5): 1512 doi: 10.1039/C8EE03727B [5] Zhou T, Shen J D, Wang Z S, et al. Regulating lithium nucleation and deposition via MOF‐derived Co@C‐modified carbon cloth for stable Li metal anode. Adv Funct Mater, 2020, 30(14): 1909159 doi: 10.1002/adfm.201909159 [6] Ponrouch A, Monti D, Boschin A, et al. Non-aqueous electrolytes for sodium-ion batteries. J Mater Chem A, 2015, 3(1): 22 doi: 10.1039/C4TA04428B [7] Gür T M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ Sci, 2018, 11(10): 2696 doi: 10.1039/C8EE01419A [8] Liu T F, Zhang Y P, Chen C, et al. Sustainability-inspired cell design for a fully recyclable sodium ion battery. Nat Commun, 2019, 10(1): 1965 doi: 10.1038/s41467-019-09933-0 [9] Xia C, Black R, Fernandes R, et al. The critical role of phase-transfer catalysis in aprotic sodium oxygen batteries. Nat Chem, 2015, 7(6): 496 doi: 10.1038/nchem.2260 [10] Wei S Y, Choudhury S, Xu J, et al. Highly stable sodiumbatteries enabled by functional ionic polymer membranes. Adv Mater, 2017, 29(12): 1605512 doi: 10.1002/adma.201605512 [11] Ma Q X, Chen Z J, Zhong S W, et al. Na-substitution induced oxygen vacancy achieving high transition metal capacity in commercial Li-rich cathode. Nano Energy, 2021, 81: 105622 doi: 10.1016/j.nanoen.2020.105622 [12] Schafzahl L, Mahne N, Schafzahl B, et al. Singlet oxygen during cycling of the aprotic sodium-O2 battery. Angew Chem Int Ed, 2017, 56(49): 15728 doi: 10.1002/anie.201709351 [13] Patrike A, Yadav P, Shelke. V, et al. Research progress and perspective on Lithium/Sodium metal anodes for next-generation rechargeable batteries. ChemSusChem, 2022, 15(14): e202200504 [14] Liu W, Sun Q, Yang Y, et al. An enhanced electrochemical performance of a sodium-air battery with graphene nanosheets as air electrode catalysts. Chem Commun, 2013, 49(19): 1951 doi: 10.1039/c3cc00085k [15] Hartmann P, Bender C L, Vra?ar M, et al. A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat Mater, 2013, 12(3): 228 doi: 10.1038/nmat3486 [16] Zhang Z J, Chen Y F, Sun S H, et al. Recent progress on three-dimensional nanoarchitecture anode materials for lithium/sodium storage. J Mater Sci Technol, 2022, 119: 167 doi: 10.1016/j.jmst.2021.11.074 [17] Wang H, Matios E, Luo J M, et al. Combining theories and experiments to understand the sodium nucleation behavior towards safe sodium metal batteries. Chem Soc Rev, 2020, 49(12): 3783 doi: 10.1039/D0CS00033G [18] Zhang Y, Wang C W, Pastel G, et al. 3D wettable framework for dendrite‐free alkali metal anodes. Adv Energy Mater, 2018, 8(18): 1800635 doi: 10.1002/aenm.201800635 [19] Li T J, Sun J C, Gao S Z, et al. Superior sodium metal anodes enabled by sodiophilic carbonized coconut framework with 3D tubular structure. Adv Energy Mater, 2021, 11(7): 2003699 doi: 10.1002/aenm.202003699 [20] Ma C Y, Xu T T, Wang Y. Advanced carbon nanostructures for future high performance sodium metal anodes. Energy Storage Mater, 2020, 25: 811 doi: 10.1016/j.ensm.2019.09.007 [21] Zeng L Y, Zhou T, Xu X J, et al. General construction of lithiophilic 3D skeleton for dendrite-free lithium metal anode via a versatile MOF-derived route. Sci China Mater, 2022, 65(2): 337 doi: 10.1007/s40843-021-1764-x [22] He W X, Zuo S Y, Xu X J, et al. Challenges and strategies of zinc anode for aqueous zinc-ion batteries. Mater Chem Front, 2021, 5(5): 2201 doi: 10.1039/D0QM00693A [23] Yao S Y, Zeng L Y, Liu J. Research progress in structure design and interface enhancement of lithium anode for high-performance lithium metal batteries. Mater Rep, 2022(16): 1姚詩言, 曾立艷, 劉軍. 高性能鋰金屬電池負極結構設計及界面強化研究進展. 材料導報, 2022(16):1 [24] Zheng X Y, Gu Z Y, Liu X Y, et al. Bridging the immiscibility of an all-fluoride fire extinguishant with highly-fluorinated electrolytes toward safe sodium metal batteries. Energy Environ Sci, 2020, 13(6): 1788 doi: 10.1039/D0EE00694G [25] Kreissl J J A, Langsdorf D, Tkachenko B A, et al. Incorporating diamondoids as electrolyte additive in the sodium metal anode to mitigate dendrite growth. ChemSusChem, 2020, 13(10): 2661 doi: 10.1002/cssc.201903499 [26] Zhao Y, Liang J W, Sun Q, et al. In situ formation of highly controllable and stable Na3PS4 as a protective layer for Na metal anode. J Mater Chem A, 2019, 7(8): 4119 doi: 10.1039/C8TA10174D [27] Zheng X Y, Fu H Y, Hu C C, et al. Toward a stable sodium metal anode in carbonate electrolyte: A compact, inorganic alloy interface. J Phys Chem Lett, 2019, 10(4): 707 doi: 10.1021/acs.jpclett.8b03536 [28] Wang H, Wang C L, Matios E, et al. Facile stabilization of the sodium metal anode with additives: Unexpected key role of sodium polysulfide and adverse effect of sodium nitrate. Angew Chem Int Ed Engl, 2018, 57(26): 7734 doi: 10.1002/anie.201801818 [29] Rakov D A, Chen F F, Ferdousi S A, et al. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat Mater, 2020, 19(10): 1096 doi: 10.1038/s41563-020-0673-0 [30] Chen Q W, He H, Hou Z, et al. Building an artificial solid electrolyte interphase with high-uniformity and fast ion diffusion for ultralong-life sodium metal anodes. J Mater Chem A, 2020, 8(32): 16232 doi: 10.1039/D0TA04715E [31] Luo W, Lin C F, Zhao O, et al. Ultrathin surface coating enables the stable sodium metal anode. Adv Energy Mater, 2017, 7(2): 1601526 doi: 10.1002/aenm.201601526 [32] Xie Y Y, Hu J X, Zhang Z A. A stable carbon host engineering surface defects for room-temperature liquid NaK anode. J Electroanal Chem, 2020, 856: 113676 doi: 10.1016/j.jelechem.2019.113676 [33] Ju Z J, Nai J W, Wang Y, et al. Biomacromolecules enabled dendrite-free lithium metal battery and its origin revealed by cryo-electron microscopy. Nat Commun, 2020, 11: 488 doi: 10.1038/s41467-020-14358-1 [34] Lee M E, Kwak H W, Kwak J H, et al. Catalytic pyroprotein seed layers for sodium metal anodes. ACS Appl Mater Interfaces, 2019, 11(13): 12401 doi: 10.1021/acsami.8b15938 [35] Hou Z, Wang W H, Yu Y K, et al. Poly(vinylidene difluoride) coating on Cu current collector for high-performance Na metal anode. Energy Storage Mater, 2020, 24: 588 doi: 10.1016/j.ensm.2019.06.026 [36] Zhai L, Yang K, Jiang F Y, et al. High-performance solid-state lithium metal batteries achieved by interface modification. J Energy Chem, 2023, 79: 357 [37] Xi L, Zhang D C, Liu J. Research progress of composite solid electrolytes for all-solid-state lithium batteries. Mater China, 2021, 40(8): 607 doi: 10.7502/j.issn.1674-3962.202012023習磊, 張德超, 劉軍. 應用于全固態鋰電池的復合固態電解質研究進展. 中國材料進展, 2021, 40(8):607 doi: 10.7502/j.issn.1674-3962.202012023 [38] Yu X W, Xue L G, Goodenough J B, et al. All-solid-state sodium batteries with a polyethylene glycol diacrylate-Na3Zr2Si2PO12 composite electrolyte. Adv Energy Sustain Res, 2021, 2(1): 2000061 doi: 10.1002/aesr.202000061 [39] Yang J, Liu G Z, Avdeev M, et al. Ultrastable all-solid-state sodium rechargeable batteries. ACS Energy Lett, 2020, 5(9): 2835 doi: 10.1021/acsenergylett.0c01432 [40] Oh J A S, Sun J G, Goh M, et al. A robust solid-solid interface using sodium-tin alloy modified metallic sodium anode paving way for all‐solid‐state battery. Adv Energy Mater, 2021, 11(32): 2101228 doi: 10.1002/aenm.202101228 [41] Liu P, Hao H, Celio H, et al. Multifunctional separator allows stable cycling of potassium metal anodes and of potassium metal batteries. Adv Mater, 2022, 34(7): e2105855 doi: 10.1002/adma.202105855 [42] Cui J Y, Wang A X, Li G J, et al. Correction: Composite sodium metal anodes for practical applications. J Mater Chem A, 2020, 8(31): 16024 doi: 10.1039/D0TA90145H [43] Liu S, Tang S, Zhang X Y, et al. Porous Al current collector for dendrite-free Na metal anodes. Nano Lett, 2017, 17(9): 5862 doi: 10.1021/acs.nanolett.7b03185 [44] Xiong W S, Xia Y, Jiang Y, et al. Highly conductive and robust three-dimensional host with excellent alkali metal infiltration boosts ultrastable lithium and sodium metal anodes. ACS Appl Mater Interfaces, 2018, 10(25): 21254 doi: 10.1021/acsami.8b03572 [45] Eng A Y S, Soni C B, Lum Y, et al. Theory-guided experimental design in battery materials research. Sci Adv, 2022, 8(19): eabm2422 doi: 10.1126/sciadv.abm2422 [46] Lu X, Luo J M, Matios E, et al. Enabling high-performance sodium metal anodes via A sodiophilic structure constructed by hierarchical Sb2MoO6 microspheres. Nano Energy, 2020, 69: 104446 doi: 10.1016/j.nanoen.2020.104446 [47] Xie Y Y, Hu J X, Han Z X, et al. Encapsulating sodium deposition into carbon rhombic dodecahedron guided by sodiophilic sites for dendrite-free Na metal batteries. Energy Storage Mater, 2020, 30: 1 doi: 10.1016/j.ensm.2020.05.008 [48] Sun B, Xiong P, Maitra U, et al. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv Mater, 2020, 32(18): e1903891 doi: 10.1002/adma.201903891 [49] Zhao Y, Adair K R, Sun X L. Recent developments and insights into the understanding of Na metal anodes for Na-metal batteries. Energy Environ Sci, 2018, 11(10): 2673 doi: 10.1039/C8EE01373J [50] Wang Y, Zhu M, Liu H X, et al. Carbon-based current collector materials for sodium metal anodes. New Carbon Mater, 2022, 37(1): 93 doi: 10.1016/S1872-5805(22)60581-X [51] Fan L L, Li X F. Recent advances in effective protection of sodium metal anode. Nano Energy, 2018, 53: 630 doi: 10.1016/j.nanoen.2018.09.017 [52] Wu F, Zhou J H, Luo R, et al. Reduced graphene oxide aerogel as stable host for dendrite-free sodium metal anode. Energy Storage Mater, 2019, 22: 376 doi: 10.1016/j.ensm.2019.02.015 [53] Wang H, Wang C L, Matios E, et al. Critical role of ultrathin graphene films with tunable thickness in enabling highly stable sodium metal anodes. Nano Lett, 2017, 17(11): 6808 doi: 10.1021/acs.nanolett.7b03071 [54] Wang A, Hu X, Tang H, et al. Processable and moldable sodium-metal anodes. Angew Chem Int Ed Engl, 2017, 56(39): 11921 doi: 10.1002/anie.201703937 [55] Lee Y, Lee J, Lee J, et al. Fluoroethylene carbonate-based electrolyte with 1 M sodium bis(fluorosulfonyl)imide enables high-performance sodium metal electrodes. ACS Appl Mater Interfaces, 2018, 10(17): 15270 doi: 10.1021/acsami.8b02446 [56] Yan k, Zhao S Q, Zhang J Q, et al. Dendrite-free sodium metal batteries enabled by the release of contact strain on flexible and sodiophilic matrix. Nano Lett, 2020, 20(8): 6112 doi: 10.1021/acs.nanolett.0c02215 [57] Liu W, Li P Y, Wang W W, et al. Directional flow-aided sonochemistry yields graphene with tunable defects to provide fundamental insight on sodium metal plating behavior. ACS Nano, 2018, 12(12): 12255 doi: 10.1021/acsnano.8b06051 [58] Wang H, Wang C L, Matios E, et al. Enabling ultrahigh rate and capacity sodium metal anodes with lightweight solid additives. Energy Storage Mater, 2020, 32: 244 doi: 10.1016/j.ensm.2020.07.021 [59] Bao C Y, Wang B, Xie Y, et al. Sodiophilic decoration of a three-dimensional conductive scaffold toward a stable Na metal anode. ACS Sustainable Chem Eng, 2020, 8(14): 5452 doi: 10.1021/acssuschemeng.9b06534 [60] Jin X, Zhao Y, Shen Z H, et al. Interfacial design principle of sodiophilicity-regulated interlayer deposition in a sandwiched sodium metal anode. Energy Storage Mater, 2020, 31: 221 doi: 10.1016/j.ensm.2020.06.040 [61] Yan J, Zhi G, Kong D Z, et al. 3D printed rGO/CNT microlattice aerogel for a dendrite-free sodium metal anode. J Mater Chem A, 2020, 8(38): 19843 doi: 10.1039/D0TA05817C [62] Kim Y J, Lee J H, Yuk S, et al. Tuning sodium nucleation and stripping by the mixed surface of carbon nanotube-sodium composite electrodes for improved reversibility. J Power Sources, 2019, 438: 227005 doi: 10.1016/j.jpowsour.2019.227005 [63] Sun B, Li P, Zhang J, et al. Dendrite-free sodium-metal anodes for high-energy sodium-metal batteries. Adv Mater, 2018, 31: e1801334 [64] Ye L, Liao M, Zhao T C, et al. A sodiophilic interphase-mediated, dendrite-free anode with ultrahigh specific capacity for sodium-metal batteries. Angew Chem Int Ed Engl, 2019, 58(47): 17054 doi: 10.1002/anie.201910202 [65] Zhao Y, Yang X F, Sun Q, et al. Dendrite-free and minimum volume change Li metal anode achieved by three-dimensional artificial interlayers. Energy Storage Mater, 2018, 15: 415 [66] Wang J J, Zhang W H, Zhang C S. Versatile fabrication of anisotropic and superhydrophobic aerogels for highly selective oil absorption. Carbon, 2019, 155: 16 doi: 10.1016/j.carbon.2019.08.049 [67] Chi S S, Qi X G, Hu Y S, et al. 3D flexible carbon felt host for highly stable sodium metal anodes. Adv Energy Mater, 2018, 8(15): 1702764 doi: 10.1002/aenm.201702764 [68] Go W, Kim M H, Park J, et al. Nanocrevasse-rich carbon fibers for stable lithium and sodium metal anodes. Nano Lett, 2019, 19(3): 1504 doi: 10.1021/acs.nanolett.8b04106 [69] Li P R, Xu T H, Ding P, et al. Highly reversible Na and K metal anodes enabled by carbon paper protection. Energy Storage Mater, 2018, 15: 8 [70] Zhao C L, Liu L L, Lu Y X, et al. Revealing an interconnected interfacial layer in solid-state polymer sodium batteries. Angew Chem Int Ed Engl, 2019, 58(47): 17026 doi: 10.1002/anie.201909877 [71] Zheng X Y, Li P, Cao Z, et al. Boosting the reversibility of sodium metal anode via heteroatom-doped hollow carbon fibers. Small, 2019, 15(41): e1902688 doi: 10.1002/smll.201902688 [72] Yoon H J, Kim N R, Jin H J, et al. Macroporous catalytic carbon nanotemplates for sodium metal anodes. Adv Energy Mater, 2018, 8(6): 1701261 doi: 10.1002/aenm.201701261 [73] Xiong W S, Jiang Y, Xia Y, et al. A robust 3D host for sodium metal anodes with excellent machinability and cycling stability. Chem Commun (Camb), 2018, 54(68): 9406 doi: 10.1039/C8CC03996H [74] Li S Y, Liu Q L, Zhou J J, et al. Hierarchical Co3O4 nanofiber-carbon sheet skeleton with superior Na/Li‐philic property enabling highly stable alkali metal batteries. Adv Funct Mater, 2019, 29(19): 1808847 doi: 10.1002/adfm.201808847 [75] Sun J C, Zhang M, Ju P, et al. Long‐life sodium metal anodes achieved by cuprous oxide-modified Ni foam host. Energy Technol, 2020, 8(3): 1901250 doi: 10.1002/ente.201901250 [76] Ye H, Wang C Y, Zuo T T, et al. Realizing a highly stable sodium battery with dendrite-free sodium metal composite anodes and O3- type cathodes. Nano Energy, 2018, 48: 369 doi: 10.1016/j.nanoen.2018.03.069 [77] Wu J X, Zou P C, Ihsan-UI-Haq M, et al. Sodiophilically graded gold coating on carbon skeletons for highly stable sodium metal anodes. Small, 2020, 16(40): e2003815 doi: 10.1002/smll.202003815 [78] Chen J Y, Xu X, He Q, et al. Advanced Current collectors for alkali metal anodes. Chem Res Chin Univ, 2020, 36(3): 386 doi: 10.1007/s40242-020-0098-y [79] Xu Y L, Menon A S, Harks P P R M L, et al. Honeycomb-like porous 3D nickel electrodeposition for stable Li and Na metal anodes. Energy Storage Mater, 2018, 12: 69 doi: 10.1016/j.ensm.2017.11.011 [80] Wang T S, Liu Y C, Lu Y X, et al. Dendrite-free Na metal plating/stripping onto 3D porous Cu hosts. Energy Storage Mater, 2018, 15: 274 [81] Zhang D, Dai A, Fan B F, et al. Three-dimensional ordered macro/mesoporous Cu/Zn as a lithiophilic current collector for dendrite-free lithium metal anode. ACS Appl Mater Interfaces, 2020, 12(28): 31542 doi: 10.1021/acsami.0c09503 [82] Yang W, Yang W, Dong L B, et al. Hierarchical ZnO nanorod arrays grown on copper foam as an advanced three-dimensional skeleton for dendrite-free sodium metal anodes. Nano Energy, 2021, 80: 105563 doi: 10.1016/j.nanoen.2020.105563 [83] Zheng X Y, Yang W J, Wang Z Q, et al. Embedding a percolated dual-conductive skeleton with high sodiophilicity toward stable sodium metal anodes. Nano Energy, 2020, 69: 104387 doi: 10.1016/j.nanoen.2019.104387 [84] He X, Jin S, Miao L C, et al. A 3D hydroxylated MXene/carbon nanotubes composite as a scaffold for dendrite‐free sodium‐metal electrodes. Angew Chem Int Ed, 2020, 59(38): 16705 doi: 10.1002/anie.202006783 [85] Fang Y Z, Lian R Q, Li H P, et al. Induction of planar sodium growth on MXene (Ti3C2Tx)-modified carbon cloth hosts for flexible sodium metal anodes. ACS Nano, 2020, 14(7): 8744 doi: 10.1021/acsnano.0c03259 [86] Luo J M, Wang C L, Wang H, et al. Pillared MXene with ultralarge interlayer spacing as a stable matrix for high performance sodium metal anodes. Adv Funct Mater, 2019, 29(3): 1805946 doi: 10.1002/adfm.201805946 -




 下載:
下載: