-
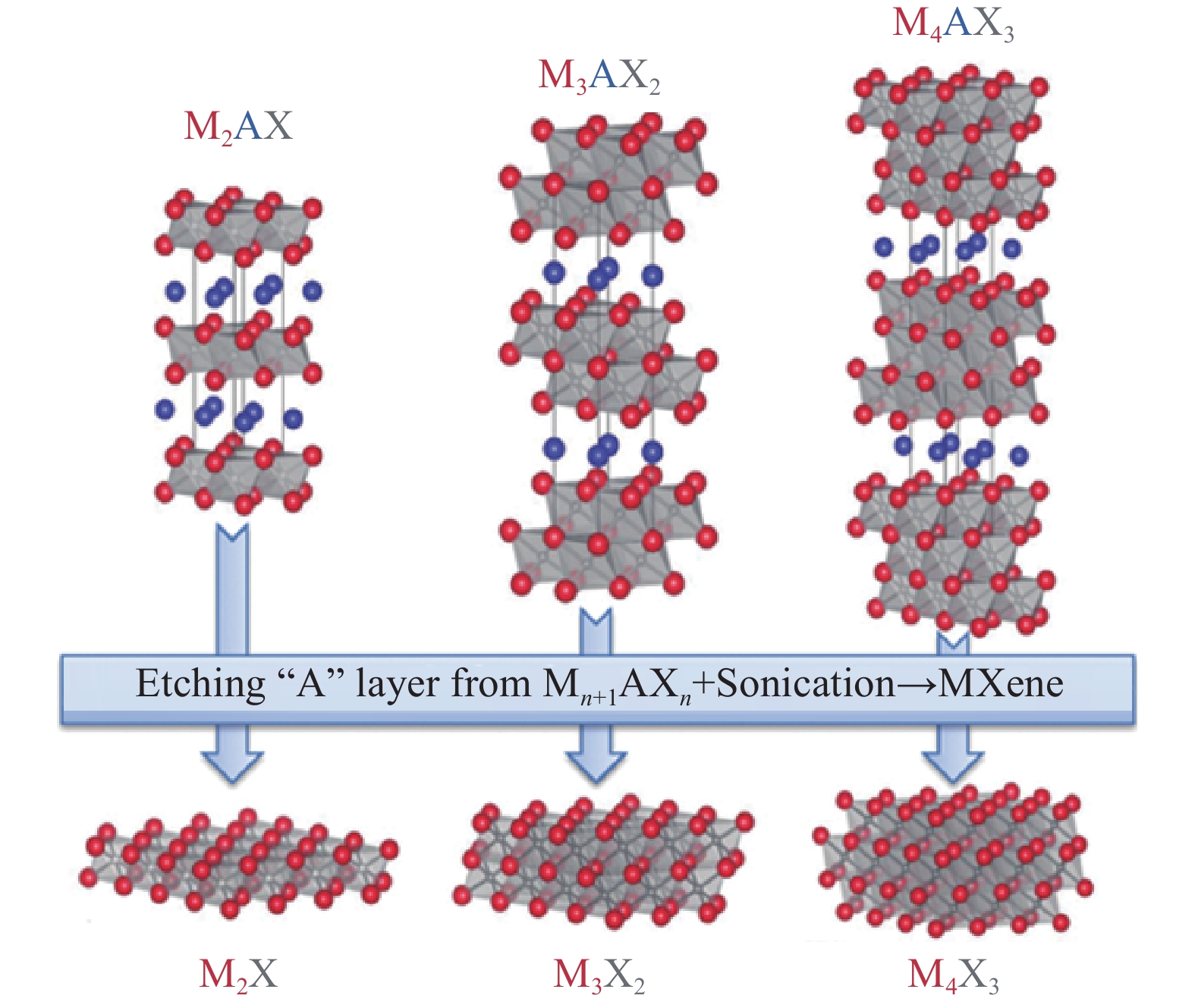
摘要: MXenes(Mn+1XnTx)是一類二維無機化合物材料,它由幾個原子層厚度的過渡金屬氮化物、碳化物或碳氮化物構成。由于具有大的比表面積、快速充放電性能和小的體積變化等優點,MXenes受到越來越多研究人員的關注。研究者希望能夠利用MXenes材料研發出具有優異電化學性能的鋰離子電池負極材料,從而提高電池的能量密度和壽命。然而MXenes材料制備過程中產生的層間堆積和坍塌限制了其進一步的發展。目前,研究人員通過將MXenes與其他材料復合制備出具有新結構的材料,不僅可以擴大層間距,改善材料結構,還有助于改進材料的電化學性能。本文介紹了MXenes與碳納米材料、過渡金屬氧化物、過渡金屬硫化物和硅等材料復合改性來提高材料電化學性能的研究策略,并探討了MXenes和堿金屬等材料復合實現穩定無枝晶的鋰離子電池金屬負極的方案。最后,闡述了MXenes應用在鋰離子電池負極材料中面臨的挑戰,并作出了展望。Abstract: MXenes are a class of two-dimensional inorganic materials comprising transition-metal carbides, nitrides, or carbonitrides of several atomic layers thick. Their general formula is (Mn+1XnTx), where M is a transition metal, such as Ti, n is the number of atomic layers, X is carbon and/or nitrogen, and Tx is the functional group introduced in the reaction process, such as OH, H, or F. They are obtained from the MAX precursor (Mn+1AXn, where A is a group of 13 or 14 elements, such as Al and Si). In 2011, Gogotsi, Barsoum, et al. first reported the synthesis of Ti3C2Tx by selective etching of the Al layer using a Ti3AlC2 MAX phase precursor impregnated with HF solution. The advantageous properties of MXenes, such as large specific surface area, fast charge–discharge performance, and small volume change, have made them attractive for lithium-ion battery anode materials, as first reported by the group Simon and Gogotsi in 2012. Since then, much attention has been paid to MXenes. Researchers hope to use MXenes for lithium-ion battery anode materials with high capacity, high safety, and improved energy density and battery life. However, a multilayer MXene material will collapse or accumulate during the preparation process, resulting in a large reduction in the contact area, thus reducing the electron and ion transport capacity of the MXene material perpendicular to the layer structure. Hence, MXenes are usually combined with other materials to improve the obtained structure, expand the layer spacing, and help enhance their electrochemical properties. This paper reviews the approaches to improving the electrochemical properties of MXenes by doping with transition-metal oxides, transition-metal sulfides, and silicon, as well as the scheme to achieve a stable and dendrite-free metal anode by using MXenes and high-capacity anode materials. Last, future challenges faced by MXenes as anode materials for lithium-ion batteries are analyzed and prospected.
-
Key words:
- MXenes /
- lithium-ion battery /
- anode material /
- lithium-ion storage /
- electrochemical performance
-
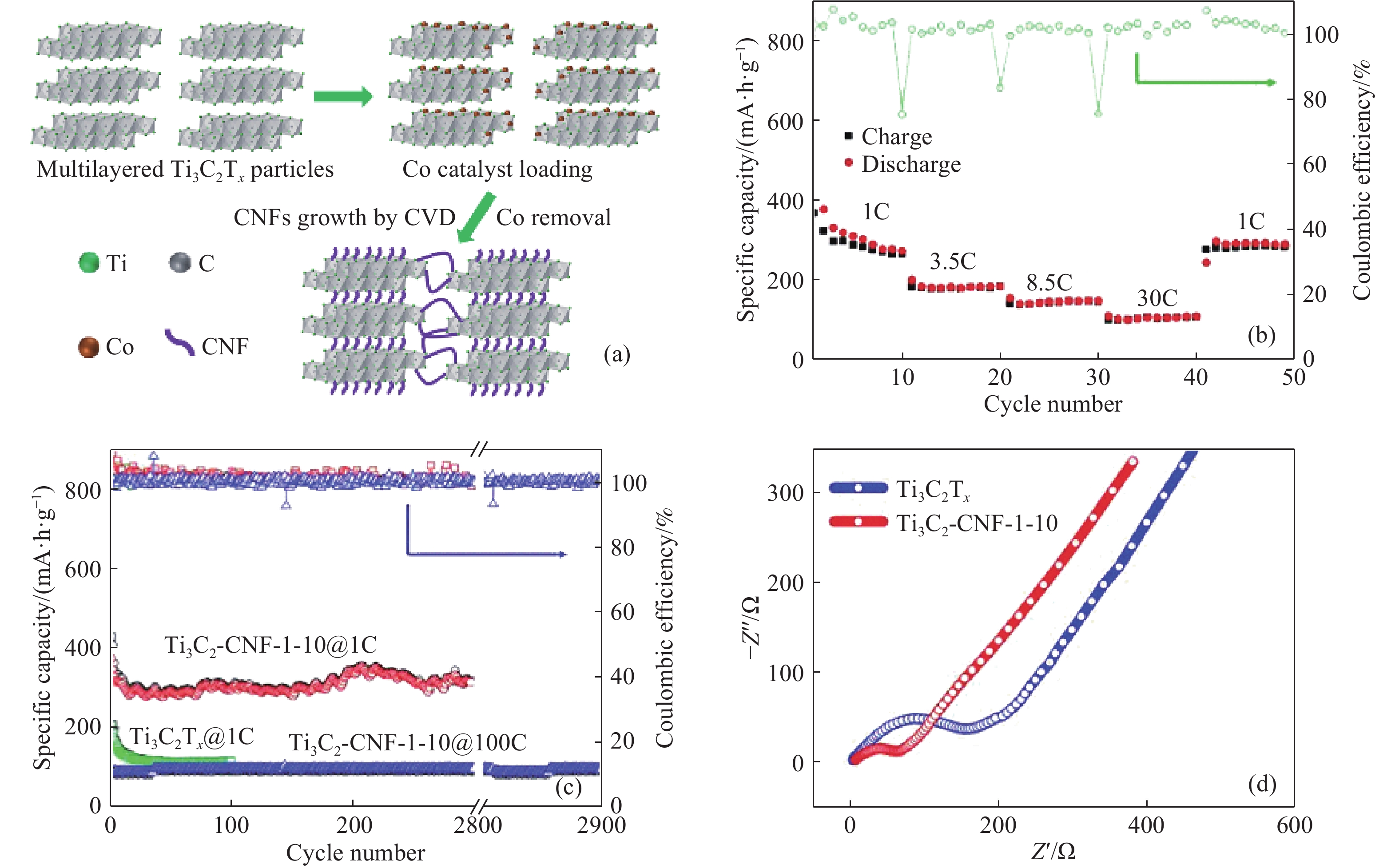
圖 3 (a) Ti3C2/CNF雜化粒子的制備示意圖;(b) Ti3C2/CNF樣品的倍率性能;(c) Ti3C2Tx樣品在1C和Ti3C2/CNF樣品在1C和100C的循環穩定性;(d) Ti3C2Tx和Ti3C2/CNF樣品的EIS圖(1C=320 mA ·g–1)[25]
Figure 3. (a) Schematic diagram of preparation of Ti3C2/CNF hybrid particles; (b) rate performance of Ti3C2/CNF samples; (c) cyclic stability of Ti3C2Tx samples at 1C and Ti3C2/CNF samples at 1C and 100C; (d) EIS diagram of Ti3C2Tx and Ti3C2/CNF samples (1C=320 mA ·g–1)[25]
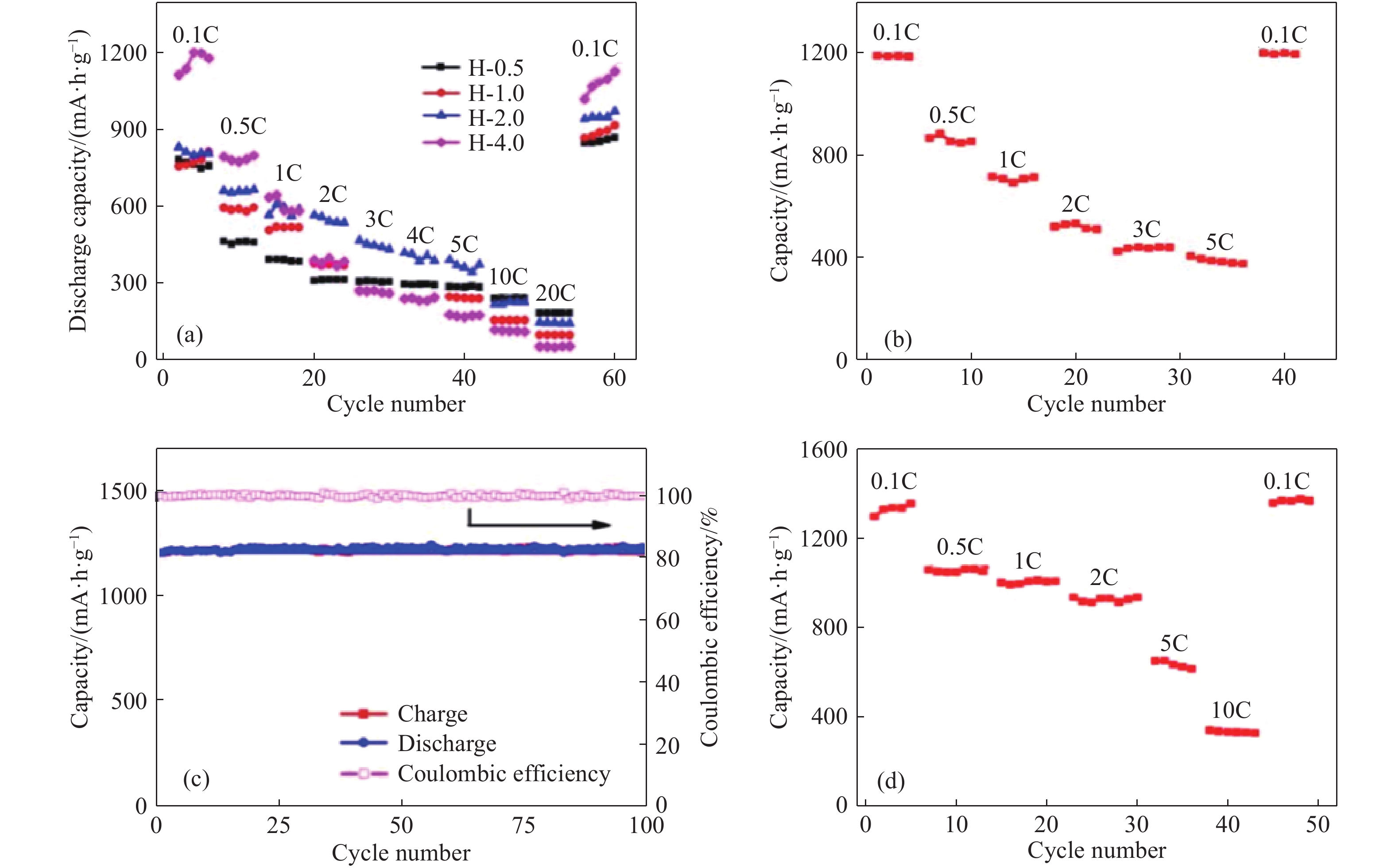
圖 4 (a) 交替過濾法制備的Ti3C2Tx/Co3O4薄膜的倍率性能;(b) 原位生長法制備的Ti3C2Tx/NiCo2O4薄膜的倍率性能;(c~d)噴涂法制備的Ti3C2Tx/Co3O4薄膜在1C下的循環性能和倍率性能(1C=320 mA ·g–1)[29]
Figure 4. (a) Rate performance of Ti3C2Tx/Co3O4 thin films prepared by alternating filtration; (b) rate performance of Ti3C2Tx/Co3O4 films prepared by in-situ growth; (c–d) properties of Ti3C2Tx/Co3O4 films prepared by sprayingcyclic properties at 1C and rate performance, respectively (1C=320 mA ·g–1)[29]
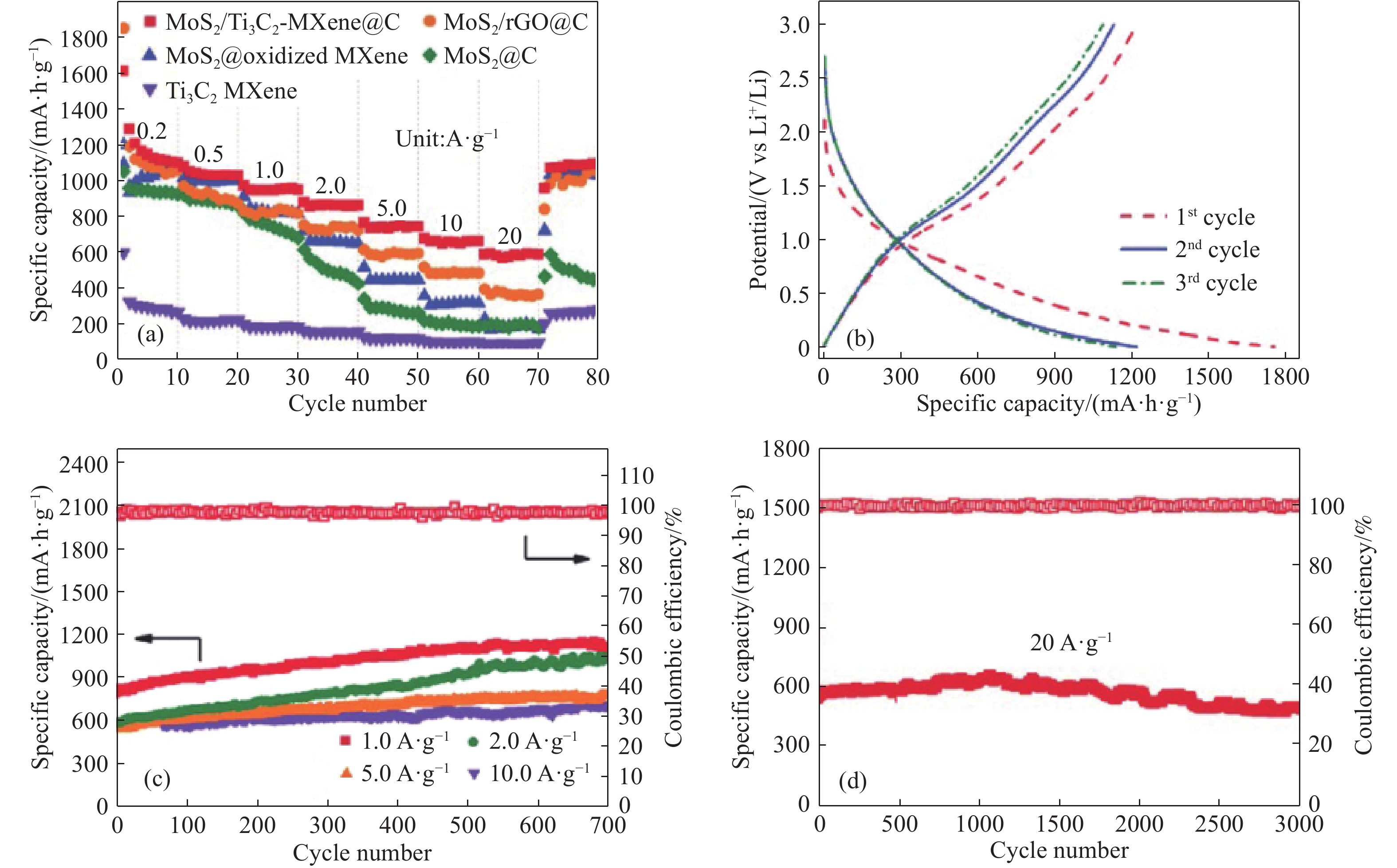
圖 5 (a) MoS2@C、MoS2/rGO@C、Ti3C2 MXene、MoS2@oxidized MXene和MoS2/Ti3C2–MXene@C電極在不同充放電電流下的倍率性能;(b) MoS2/Ti3C2–MXene@C電極在0.2 A·g–1下的充放電曲線;(c) MoS2/Ti3C2–MXene@C電極在1.0、2.0、5.0和10.0 A·g–1的循環性能;(d) MoS2/Ti3C2–MXene@C電極在高電流密度20 A·g-1下的循環性能[33]
Figure 5. (a) Rate performance of MoS2@C, MoS2/rGO@C, Ti3C2 MXene, MoS2/oxidized MXene and MoS2/Ti3C2–MXene@C electrodes at different current density; (b) discharge-charge profiles of MoS2/Ti3C2–MXene@C electrode at 0.2 A·g–1 ; (c) cyclic performance of MoS2/Ti3C2–MXene@C electrodes at the current density of 1.0, 2.0, 5.0 and 10.0 A·g–1,respectively; (d) cyclic performance of MoS2/Ti3C2–MXene@C electrodes at the current density of 20.0 A·g–1 [33]
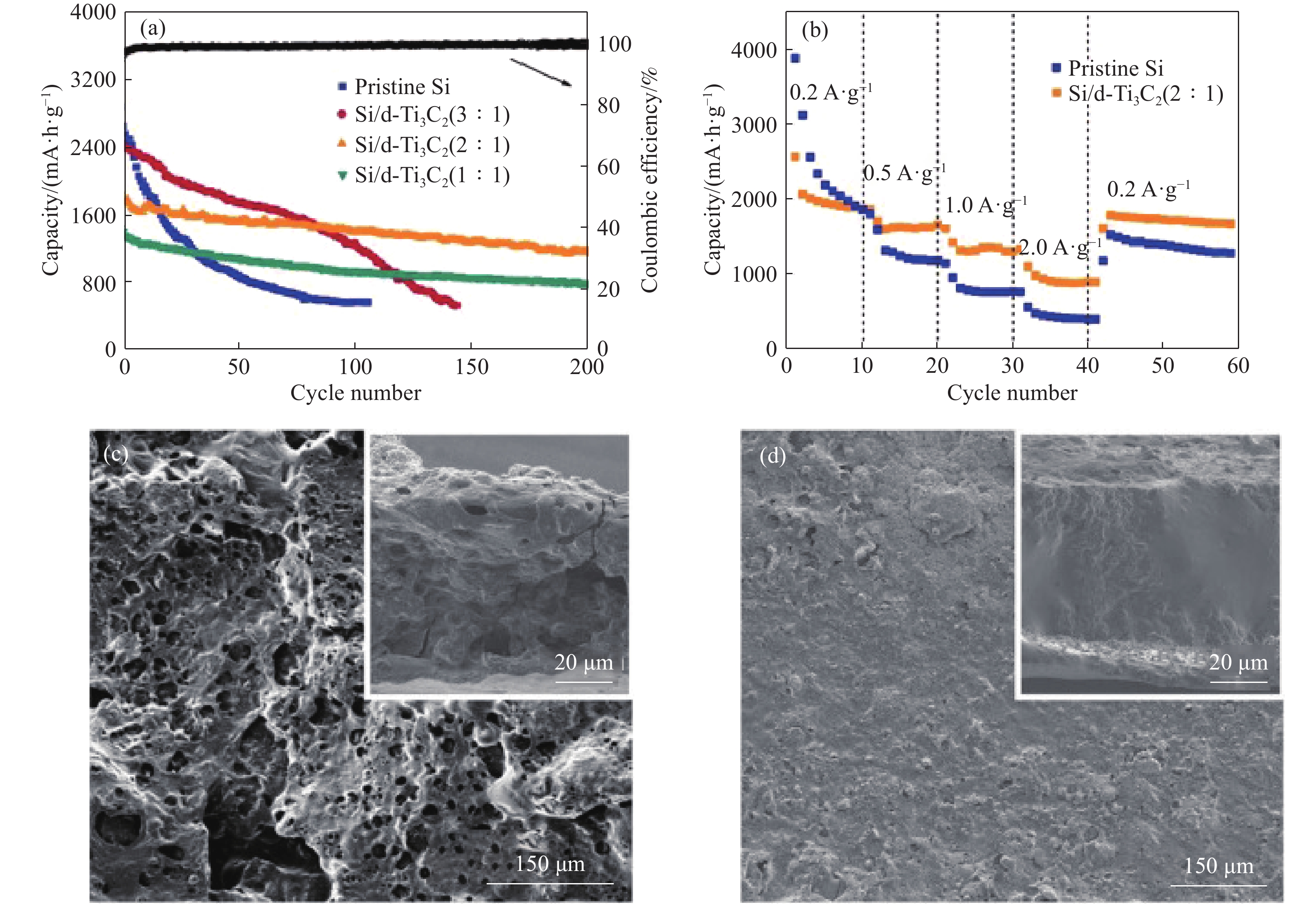
圖 6 (a) 不同質量比的Si/d-Ti3C2雜化材料在500 mA·g–1電流密度下的循環性能;(b) Si/d-Ti3C2(2∶1)雜化和原始Si納米顆粒的倍率性能;(c~d)分別為原始Si負極和Si/d-Ti3C2負極循環50次后的SEM圖像[39]
Figure 6. (a) Cyclic properties of Si/d-Ti3C2 hybrid materials with different mass ratios at the current density of 500 mA·g–1; (b) Rate performance of Si/d-Ti3C2(2∶1) hybrid and original Si nanoparticles; (c–d) SEM images of the original Si anode and Si/d-Ti3C2 anode after 50 cycles, respectively[39]
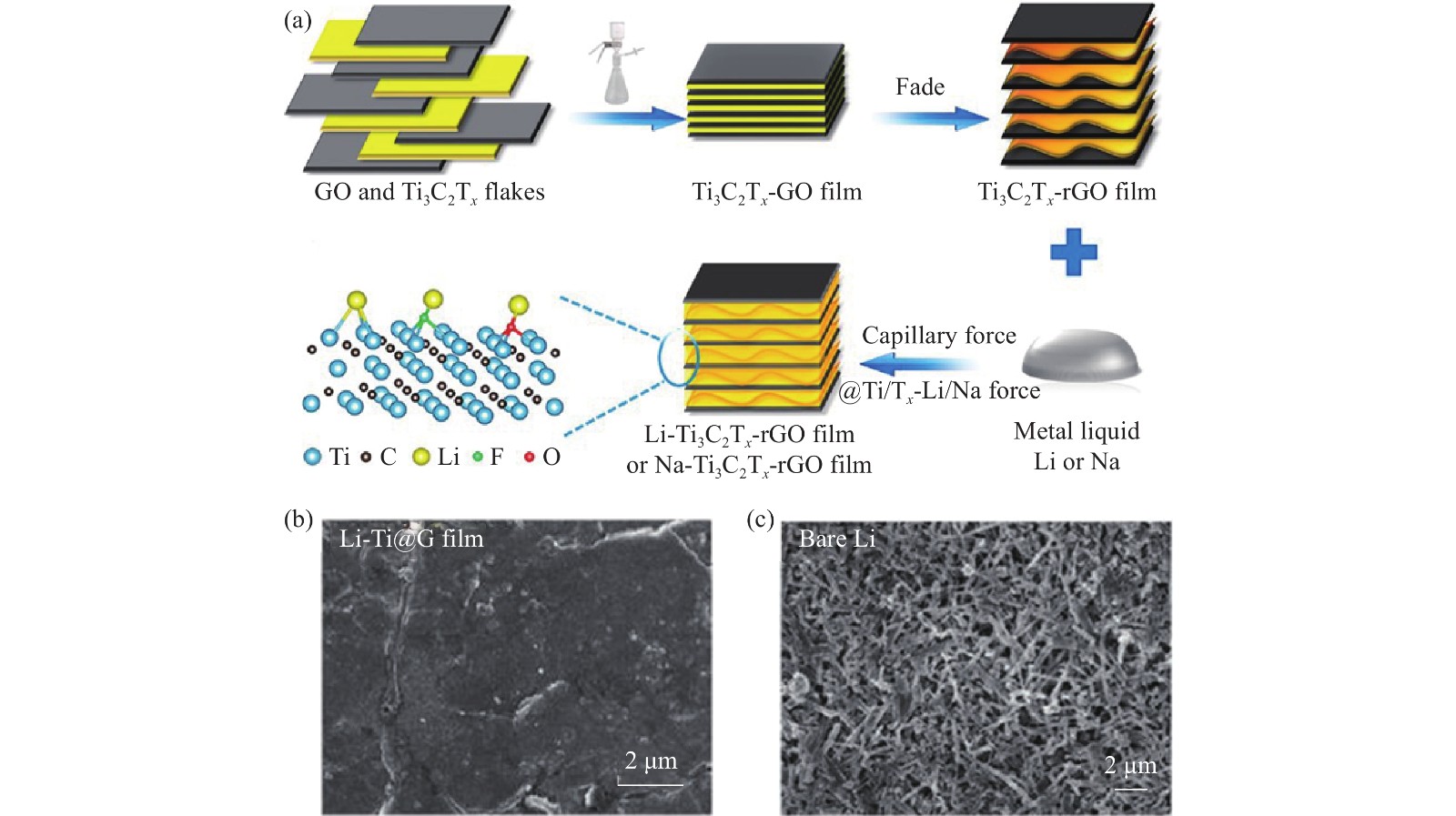
圖 7 (a) Li-Ti3C2Tx-rGO復合負極制備過程示意圖;(b~c) Li-Ti3C2Tx-rGO復合負極和裸鋰負極在1 mA·cm–2循環200 h后的SEM圖像[45]
Figure 7. (a) Schematic diagram of preparation process of Li-Ti3C2Tx-rGO composite anode; (b–c) SEM images of Li-Ti3C2Tx-rGO composite anode and bare lithium anode at the current density of 1 mA·cm–2 after cycling 200 h[45]
表 1 MXenes負極材料的電化學性能
Table 1. Electrochemical properties of MXenes materials
MXene type Current density/
(mA·g–1)Initial discharge
capacity/
(mA·h·g–1)Cycle
numberSpecific capacity after
cycling/
(mA·h·g–1)Ref V2C 370 467 20 260 [46] MoS2/Ti3C2 500 — 50 230 [47] Nb4C3Tx 1000 116 100 320 [48] CNTs@ Ti3C2 1000 479 250 445 [49] Na0.23TiO2/Ti3C2 5000 — 4000 178 [50] MXene/Si@SiOx@C 42000 510 1000 390 [51] Sn/SnOx@Ti3C2 50 1981.3 200 594.2 [52] Si p-NS@TNSs 200 2588 150 1154 [53] Ti3C2Tx/Fe3O4 100 565.3 100 450 [54] V4C3-MXene/MoS2/C 1000 933.7 450 622.6 [55] S-TC-2 100 1348 100 462.3 [35] N-S-VCT 2000 — 300 298 [56] nMH/MX-60 100 1051.2 100 389.3 [57] SnO2–Ti2C–C 2000 — 500 763.18 [58] Ti3C2@VO2 100 1107.8 100 365.6 [59] Silicon/MXene 200 — 100 2118 [60] CoP–Co2P/Ti3C2 700 — 1000 650 [61] www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Lin Z F, Shao H, Xu K, et al. MXenes as high-rate electrodes for energy storage. Trends Chem, 2020, 2(7): 654 doi: 10.1016/j.trechm.2020.04.010 [2] Li M, Huang Q. Recent progress and prospects of ternary layered carbides/nitrides MAX phases and their derived two-dimensional nanolaminates MXenes. J Inorg Mater, 2020, 35(1): 1李勉, 黃慶. 三元層狀碳氮化合物(MAX 相)及其衍生二維納米材料(MXene)研究趨勢與展望. 無機材料學報, 2020, 35(1):1 [3] Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater, 2011, 23: 4248 doi: 10.1002/adma.201102306 [4] Pang J B, Mendes R G, Bachmatiuk A, et al. Applications of 2D MXenes in energy conversion and storage systems. Chem Soc Rev, 2019, 48(1): 72 doi: 10.1039/C8CS00324F [5] Magnuson M, Mattesini M. Chemical bonding and electronic-structure in MAX phases as viewed by X-ray spectroscopy and density functional theory. Thin Solid Films, 2017, 621: 108 doi: 10.1016/j.tsf.2016.11.005 [6] Ghidiu M, Lukatskaya M R, Zhao M Q, et al. Conductive two-dimensional titanium carbide 'clay' with high volumetric capacitance. Nature, 2014, 516(7529): 78 doi: 10.1038/nature13970 [7] Liu F F, Zhou A G, Chen J F, et al. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl Surf Sci, 2017, 416: 781 doi: 10.1016/j.apsusc.2017.04.239 [8] Alhabeb M, Maleski K, Mathis T S, et al. Selective etching of silicon from Ti3SiC2(MAX) to obtain 2D titanium carbide (MXene). Angew Chem Int Ed, 2018, 57(19): 5444 doi: 10.1002/anie.201802232 [9] Li T F, Yao L L, Liu Q L, et al. Fluorine-free synthesis of high-purity Ti3C2Tx(T=OH, O) via alkali treatment. Angew Chem Int Ed, 2018, 57(21): 6115 doi: 10.1002/anie.201800887 [10] Li Y B, Shao H, Lin Z F, et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat Mater, 2020, 19(8): 894 doi: 10.1038/s41563-020-0657-0 [11] Persson P O ?, Rosen J. Current state of the art on tailoring the MXene composition, structure, and surface chemistry. Curr Opin Solid State Mater Sci, 2019, 23(6): 100774 doi: 10.1016/j.cossms.2019.100774 [12] Qi X, Chen X, Peng S K, et al. Research progress on two-dimensional nanomaterials MXenes and their application for lithium-ion batteries. J Mater Eng, 2019, 47(12): 10齊新, 陳翔, 彭思侃, 等. MXenes二維納米材料及其在鋰離子電池中的應用研究進展. 工程科學學報, 2019, 47(12):10 [13] Anasori B, Lukatskaya M R, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater, 2017, 2(2): 16098 doi: 10.1038/natrevmats.2016.98 [14] Zheng W, Zhang P G, Tian W B, et al. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater Chem Phys, 2018, 206: 270 doi: 10.1016/j.matchemphys.2017.12.034 [15] Hart J L, Hantanasirisakul K, Lang A C, et al. Control of MXenes’ electronic properties through termination and intercalation. Nat Commun, 2019, 10: 522 doi: 10.1038/s41467-018-08169-8 [16] Lee Y, Kim S J, Kim Y J, et al. Oxidation-resistant titanium carbide MXene films. J Mater Chem A, 2020, 8(2): 573 doi: 10.1039/C9TA07036B [17] Persson I, Halim J, Hansen T W, et al. How much oxygen can a MXene surface take before it breaks? Adv Funct Mater, 2020, 30(47): 1909005 [18] Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev, 2004, 104(10): 4303 doi: 10.1021/cr030203g [19] Naguib M, Come J, Dyatkin B, et al. MXene: a promising transition metal carbide anode for lithium-ion batteries. Electrochem Commun, 2012, 16(1): 61 doi: 10.1016/j.elecom.2012.01.002 [20] Mashtalir O, Naguib M, Mochalin V N, et al. Intercalation and delamination of layered carbides and carbonitrides. Nat Commun, 2013, 4: 1716 doi: 10.1038/ncomms2664 [21] Tang Q, Zhou Z, Shen P W. Are MXenes promising anode materials for Li-ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J Am Chem Soc, 2012, 134(40): 16909 doi: 10.1021/ja308463r [22] Sun D D, Wang M S, Li Z Y, et al. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem Commun, 2014, 47: 80 doi: 10.1016/j.elecom.2014.07.026 [23] Xie Y, Naguib M, Mochalin V N, et al. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J Am Chem Soc, 2014, 136(17): 6385 doi: 10.1021/ja501520b [24] Ren C G, Zhao M Q, Makaryan T, et al. Porous two-dimensional transition metal carbide (MXene) flakes for high-performance Li-ion storage. ChemElectrochem, 2016, 3(5): 689 doi: 10.1002/celc.201600059 [25] Lin Z Y, Sun D F, Huang Q, et al. Carbon nanofiber bridged two-dimensional titanium carbide as a superior anode for lithium-ion batteries. J Mater Chem A, 2015, 3(27): 14096 doi: 10.1039/C5TA01855B [26] Liu D R, Wang L B, He Y, et al. Enhanced reversible capacity and cyclic performance of lithium-ion batteries using SnO2 interpenetrated MXene V2C architecture as anode materials. Energy Technol, 2021, 9(2): 2000753 doi: 10.1002/ente.202000753 [27] Seok D, Shin W H, Kang S W, et al. Piezoelectric composite of BaTiO3-coated SnO2 microsphere: Li-ion battery anode with enhanced electrochemical performance based on accelerated Li+ mobility. J Alloys Compd, 2021, 870: 159267 doi: 10.1016/j.jallcom.2021.159267 [28] Zhang C F, Kim S J, Ghidiu M, et al. Layered orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx hierarchical composites for high performance Li-ion batteries. Adv Funct Mater, 2016, 26(23): 4143 doi: 10.1002/adfm.201600682 [29] Zhao M Q, Torelli M, Ren C E, et al. 2D titanium carbide and transition metal oxides hybrid electrodes for Li-ion storage. Nano Energy, 2016, 30: 603 doi: 10.1016/j.nanoen.2016.10.062 [30] Ahmed B, Anjum D H, Gogotsi Y, et al. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy, 2017, 34: 249 doi: 10.1016/j.nanoen.2017.02.043 [31] Rakhi R B, Ahmed B, Hedhili M N, et al. Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem Mater, 2015, 27(15): 5314 doi: 10.1021/acs.chemmater.5b01623 [32] Wang H, Feng H B, Li J H. Graphene and graphene-likelLayered transition metal dichalcogenides in energy conversion and storage. Small, 2014, 10(11): 2165 doi: 10.1002/smll.201303711 [33] Wu X, Wang Z, Yu M, et al. Stabilizing the MXenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv Mater, 2017, 29(24): 1607017 doi: 10.1002/adma.201607017 [34] Jin J, Xiao T, Zhang Y F, et al. Hierarchical MXene/transition metal chalcogenide heterostructures for electrochemical energy storage and conversion. Nanoscale, 2021, 13(47): 19740 doi: 10.1039/D1NR05799E [35] Li J F, Han L, Li Y Q, et al. MXene-decorated SnS2/Sn3S4 hybrid as anode material for high-rate lithium-ion batteries. Chem Eng J, 2020, 380: 122590 doi: 10.1016/j.cej.2019.122590 [36] Wang A N, Chen Y X, Liu L, et al. Sulfur nanoparticles/Ti3C2Tx MXene with an optimum sulfur content as a cathode for highly stable lithium-sulfur batteries. Dalton Trans, 2021, 50(16): 5574 doi: 10.1039/D1DT00381J [37] Feng K, Li M, Liu W, et al. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small, 2018, 14(8): 1702737 doi: 10.1002/smll.201702737 [38] Casimir A, Zhang H G, Ogoke O, et al. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy, 2016, 27: 359 doi: 10.1016/j.nanoen.2016.07.023 [39] Zhu X Q, Shen J L, Chen X F, et al. Enhanced cycling performance of Si-MXene nanohybrids as anode for high performance lithium ion batteries. Chem Eng J, 2019, 378: 122212 doi: 10.1016/j.cej.2019.122212 [40] An Y L, Tian Y, Zhang Y C, et al. Two-dimensional silicon/carbon from commercial alloy and CO2 for lithium storage and flexible Ti3C2Tx MXene-based lithium-metal batteries. ACS Nano, 2020, 14(12): 17574 doi: 10.1021/acsnano.0c08336 [41] An Y L, Tian Y, Wei H, et al. Porosity- and graphitization-controlled fabrication of nanoporous dilicon@carbon for lithium storage and its conjugation with MXene for lithium-metal anode. Adv Funct Mater, 2020, 30(9): 1908721 doi: 10.1002/adfm.201908721 [42] Cao R G, Xu W, Lv D P, et al. Anodes for rechargeable lithium-sulfur batteries. Adv Energy Mater, 2015, 5(16): 1402273 doi: 10.1002/aenm.201402273 [43] Tian Y, An Y L, Wei C L, et al. Flexible and free-standing Ti3C2Tx MXene@Zn paper for dendrite-free aqueous Zinc metal batteries and nonaqueous lithium metal batteries. ACS Nano, 2019, 13(10): 11676 doi: 10.1021/acsnano.9b05599 [44] Lin D C, Liu Y Y, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotechnol, 2017, 12(3): 194 doi: 10.1038/nnano.2017.16 [45] Fang Y Z, Zhang Y, Zhu K, et al. Lithiophilic three-dimensional porous Ti3C2Tx-rGO membrane as a stable scaffold for safe alkali metal (Li or Na) anodes. ACS Nano, 2019, 13(12): 14319 doi: 10.1021/acsnano.9b07729 [46] Liu F F, Zhou J, Wang S W, et al. Preparation of high-purity V2C MXene and electrochemical properties as Li-ion batteries. J Electrochem Soc, 2017, 164(4): A709 doi: 10.1149/2.0641704jes [47] Zheng M, Guo R S, Liu Z C, et al. MoS2 intercalated p-Ti3C2 anode materials with sandwich-like three dimensional conductive networks for lithium-ion batteries. J Alloys Compd, 2018, 735: 1262 doi: 10.1016/j.jallcom.2017.11.250 [48] Zhao S S, Meng X, Zhu K, et al. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy Storage Mater, 2017, 8: 42 doi: 10.1016/j.ensm.2017.03.012 [49] Zheng W, Zhang P, Chen J, et al. In situ synthesis of CNTs@Ti3C2 hybrid structures by microwave irradiation for high-performance anodes in lithium ion batteries. J Mater Chem A, 2018, 6(8): 3543 doi: 10.1039/C7TA10394H [50] Huang J M, Meng R J, Zu L H, et al. Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries. Nano Energy, 2018, 46: 20 [51] Zhang Y L, Mu Z J, Lai J P, et al. MXene/Si@SiOx@C Layer-by-layer superstructure with autoadjustable function for superior stable lithium storage. ACS Nano, 2019, 13(2): 2167 [52] Zuo D C, Song S C, An C S, et al. Synthesis of sandwich-like structured Sn/SnOx@MXene composite through in-situ growth for highly reversible lithium storage. Nano Energy, 2019, 62: 401 doi: 10.1016/j.nanoen.2019.05.062 [53] Xia M T, Chen B J, Gu F, et al. Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance. ACS Nano, 2020, 14(4): 5111 doi: 10.1021/acsnano.0c01976 [54] Jiang F Y, Du R, Yan X S, et al. Ferroferric oxide nanoclusters decorated Ti3C2Tx nanosheets as high performance anode materials for lithium ion batteries. Electrochimca Acta, 2020, 329: 135146 doi: 10.1016/j.electacta.2019.135146 [55] Bai J, Zhao B C, Lin S, et al. Construction of hierarchical V4C3-MXene/MoS2/C nanohybrids for high rate lithium-ion batteries. Nanoscale, 2020, 12(2): 1144 doi: 10.1039/C9NR07646H [56] Zhang Y J, Li J L, Gong Z W, et al. Nitrogen and sulfur co-doped vanadium carbide MXene for highly reversible lithium-ion storage. J Colloid Interface Sci, 2021, 587: 489 doi: 10.1016/j.jcis.2020.12.044 [57] Zhong S L, Ju S L, Shao Y F, et al. Magnesium hydride nanoparticles anchored on MXene sheets as high capacity anode for lithium-ion batteries. J Energy Chem, 2021, 62: 431 doi: 10.1016/j.jechem.2021.03.049 [58] Zhu M H, Deng X Q, Ke J, et al. Graphite nano-modified SnO2–Ti2C MXene as anode material for high-performance lithium-ion batteries. J Alloys Compd, 2021, 886: 161139 doi: 10.1016/j.jallcom.2021.161139 [59] Wang Z T, Wang R C, Tang L B, et al. A sandwich-like Ti3C2@VO2 composite synthesized by a hydrothermal method for lithium storage. Solid State Ion, 2021, 369: 115714 doi: 10.1016/j.ssi.2021.115714 [60] Tian Y, An Y L, Feng J K. Flexible and freestanding silicon/MXene composite papers for high-performance lithium-ion batteries. ACS Appl Mater Interfaces, 2019, 11(10): 10004 doi: 10.1021/acsami.8b21893 [61] Wang Z J, Wang F, Liu K Y. Cobalt phosphide nanoparticles grown on Ti3C2 nanosheet for enhanced lithium ions storage performances. J Alloys Compd, 2021, 853: 157136 doi: 10.1016/j.jallcom.2020.157136 -




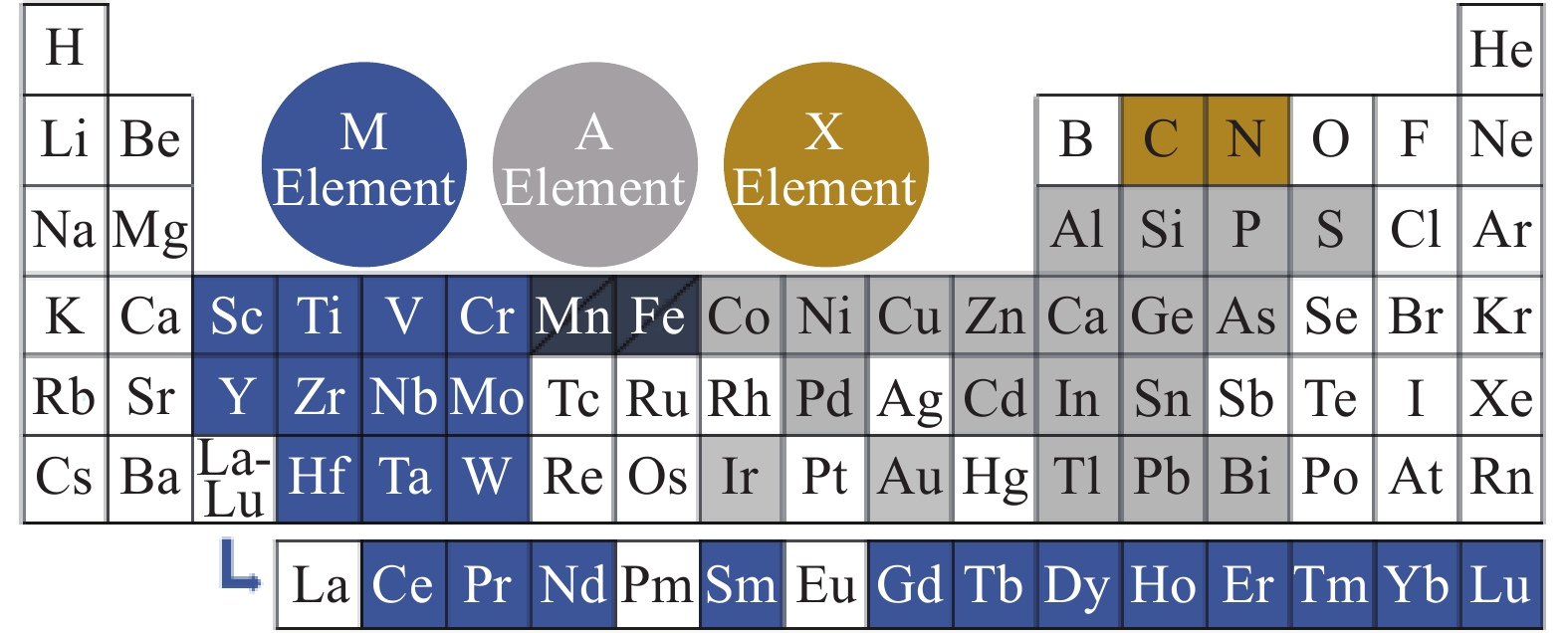
 下載:
下載: