Research progress in anode transition metal-based catalysts for direct methanol fuel cell
-
摘要: 發展可替代能源對緩解全球能源問題具有重要意義。直接甲醇燃料電池(DMFC)因其工作溫度低、能量密度高以及低污染物排放特性正逐漸成為最有發展前景的便攜式能源技術之一。目前,其商業化進程主要取決于陽極甲醇氧化反應(MOR)的動力學快慢。貴金屬作為最常用的陽極催化劑得到了廣泛的研究,但是其稀缺性以及易受COads中間產物中毒影響限制了其應用。考慮到以上問題,具有優異抗中毒能力的低Pt或者非Pt納米催化劑的設計和研發變得十分重要。本文從DMFC陽極電催化原理出發,總結了過渡金屬基催化劑(過渡金屬?貴金屬催化劑、過渡金屬催化劑以及自支撐催化劑)在MOR中的研究進展。重點強調了納米催化劑的組成成分、多孔結構、高指數面、晶體缺陷以及頂點增強效應等對其電化學性能的影響。最后,展望了過渡金屬基電催化劑在DMFC中所面臨的機遇和挑戰。Abstract: The development of alternative energy resources is of great significance to alleviate the global energy issue. The direct methanol fuel cell (DMFC) is gradually becoming one of the most promising portable energy technologies due to the merits of low operating temperature, high energy density, and low pollutant emission. Currently, its commercialization process mainly depends on the kinetics of the anodic methanol oxidation reaction (MOR). Noble metals have been widely studied as the most commonly used anode catalysts. However, high prices and limited reserves have severely hindered their further development. In addition, the active surface of Pt is susceptible to the poison of COads intermediate products, leading to the rapid loss of the catalytic activity due to blocked Pt sites. Considering the above problems, the design and development of low Pt or non-Pt nanocatalysts with an excellent antipoisoning ability have become very important. Transition metals have been widely used as promising substitutes for noble metal catalysts because of their abundant reserves, low price, and high catalytic activity. Among the transition metals studied, Ni, Cu, and Co have attracted sustained attention because of their high corrosion resistance. Owing to the ligand effect and synergistic effect, the addition of transition metals can effectively weaken the adsorption of COads intermediates on Pt sites. At the same time, non-noble transition metals are easy to form MOOH active species, which promote the oxidation of COads intermediates. Besides, methanol electrooxidation performance is closely related to the shape, structure, and composition of transition metals. From the principle of DMFC anode electrocatalysis, this review summarized the research progress of transition metal-based catalysts (transition metal-noble metal catalysts, transition metal catalysts, and self-supporting catalysts) in MOR. More importantly, the effects of the nanocatalyst composition, porous structure, high-index surface, crystal defects, and vertex enhancement on its electrochemical properties were emphasized. Finally, opportunities and challenges faced by transition metal-based electrocatalysts in DMFC were discussed.
-
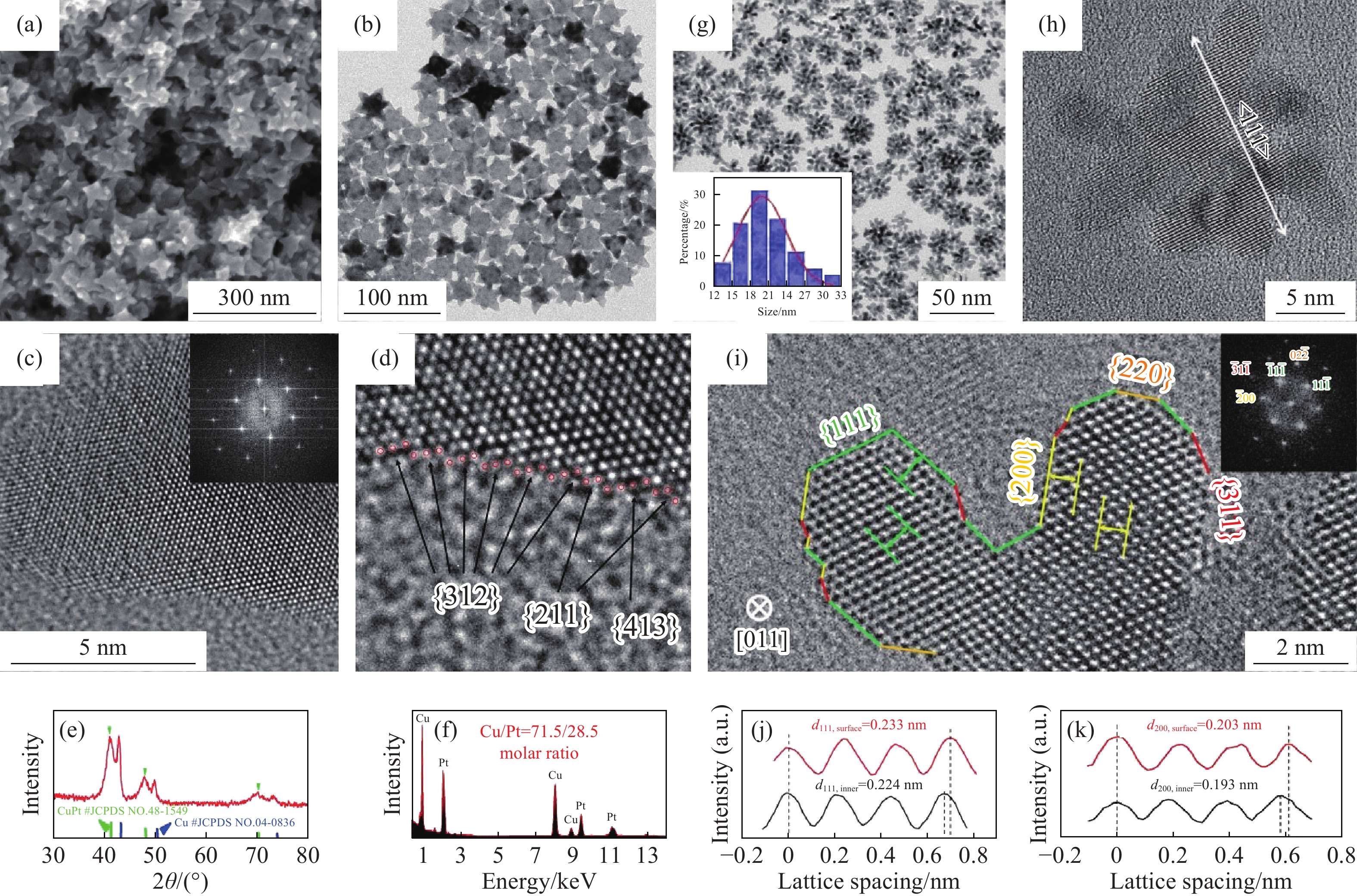
圖 2 CuPt納米晶的(a)掃描電鏡和(b)透射電鏡圖像;(c)沿[111]帶軸的高分辨透射電鏡圖像(插圖:傅里葉變換圖像);(d)高指數晶面{211}、{312}和{413};(e)X射線衍射譜;(f)透射電鏡能譜[28];(g)Ni0.20Pt0.80納米花的透射電鏡圖像,插圖:尺寸分布直方圖;(h){111}面的高分辨電鏡圖像;(i)沿[011]軸觀察到的高分辨電鏡圖像,插圖:傅里葉變換圖像;沿(j){111}和(k){200}面原子層的線強度分布[30]
Figure 2. (a) Scanning electron microscopy and (b) transmission electron microscopy images of the CuPt nanocrystal; (c) high-resolution transmission electron microscopy image along the [111] zone axis (inset: fast Fourier transforms pattern); (d) high-index surface {211}, {312}, and {413}; (e) X-ray diffraction pattern and (f) transmission electron microscopy energy-dispersive X-ray spectroscopy [28]; (g) transmission electron microscopy image of Ni0.20Pt0.80 nanoflowers; inset: size distribution histogram; (h) high-resolution transmission electron microscopy image with {111} facets; (i) high-resolution transmission electron microscopy image viewed along the [011] axis (inset: fast Fourier transforms pattern); line intensity curves along the atomic layers of (j) {111} and (k) {200} facets[30]
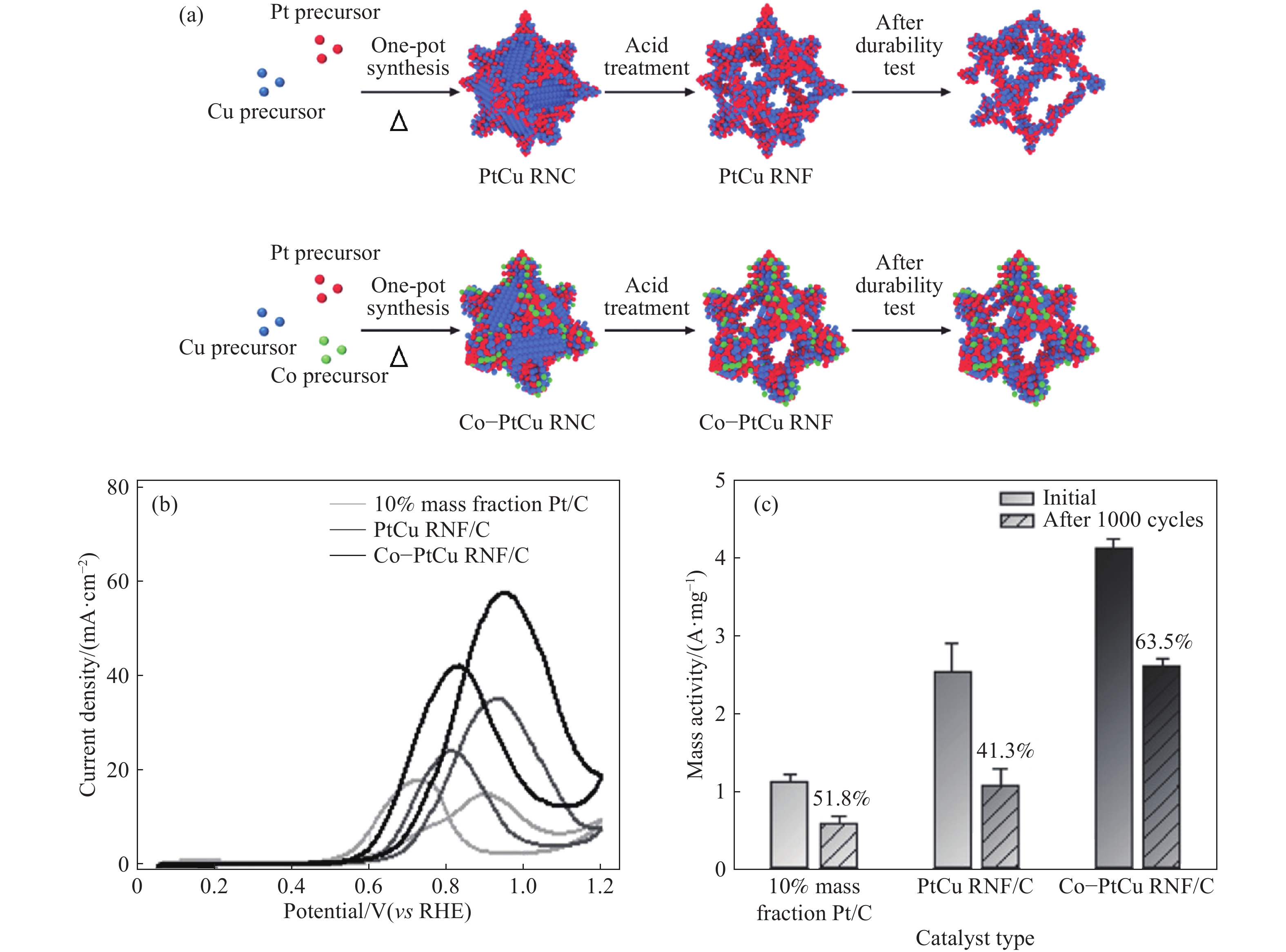
圖 4 (a)PtCu RNF和Co-PtCu RNF形成過程示意圖;(b)催化劑在0.1 mol·L?1 HClO4 + 1 mol·L?1 CH3OH溶液中的循環伏安曲線;(c)加速耐久性實驗前后甲醇氧化反應的質量活性[41]
Figure 4. (a) Schematic of forming PtCu RNF and Co-PtCu RNF; (b) CVs of catalysts in 0.1 mol·L?1 HClO4 + 1 mol·L?1 CH3OH solution; (c) mass activity for the methanol oxidation reaction before and after accelerated durability tests[41]
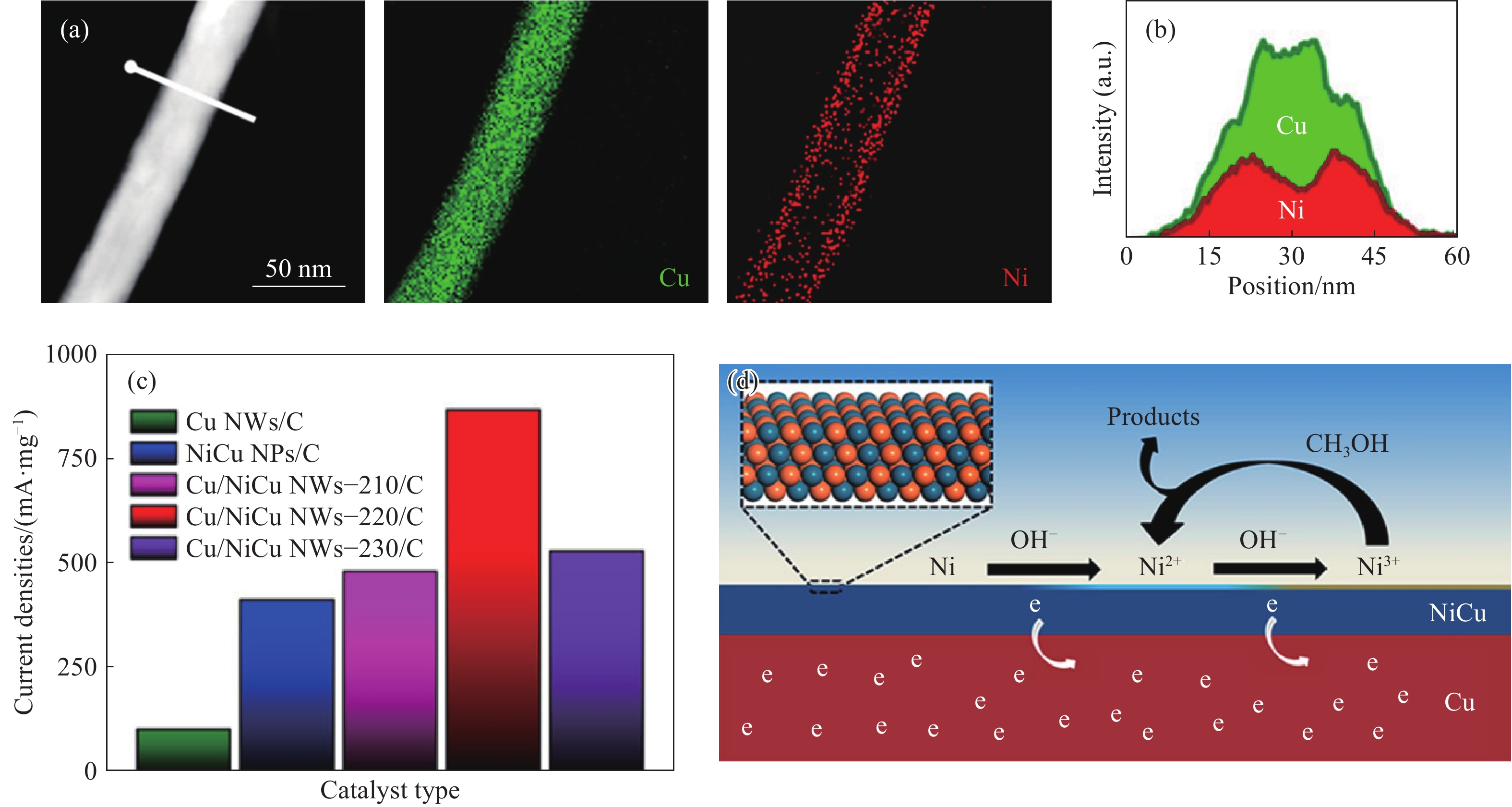
圖 6 (a)Ni和Cu的高角環形暗場像-掃描透射電子圖像以及元素分布圖像;(b)Cu/NiCu NWs?220的能譜線掃描;(c)催化劑在1.55 V (vs RHE)下的質量電流密度;(d)甲醇在Cu/NiCu NWs表面的氧化機理[45]
Figure 6. (a) HAADF-STEM image and elemental mapping images for Cu and Ni; (b) EDS line scan profile for Cu/NiCu NWs?220; (c) mass current densities at 1.55 V (vs RHE) for the prepared catalysts; (d) methanol oxidation mechanism on the surface of Cu/NiCu NWs[45]
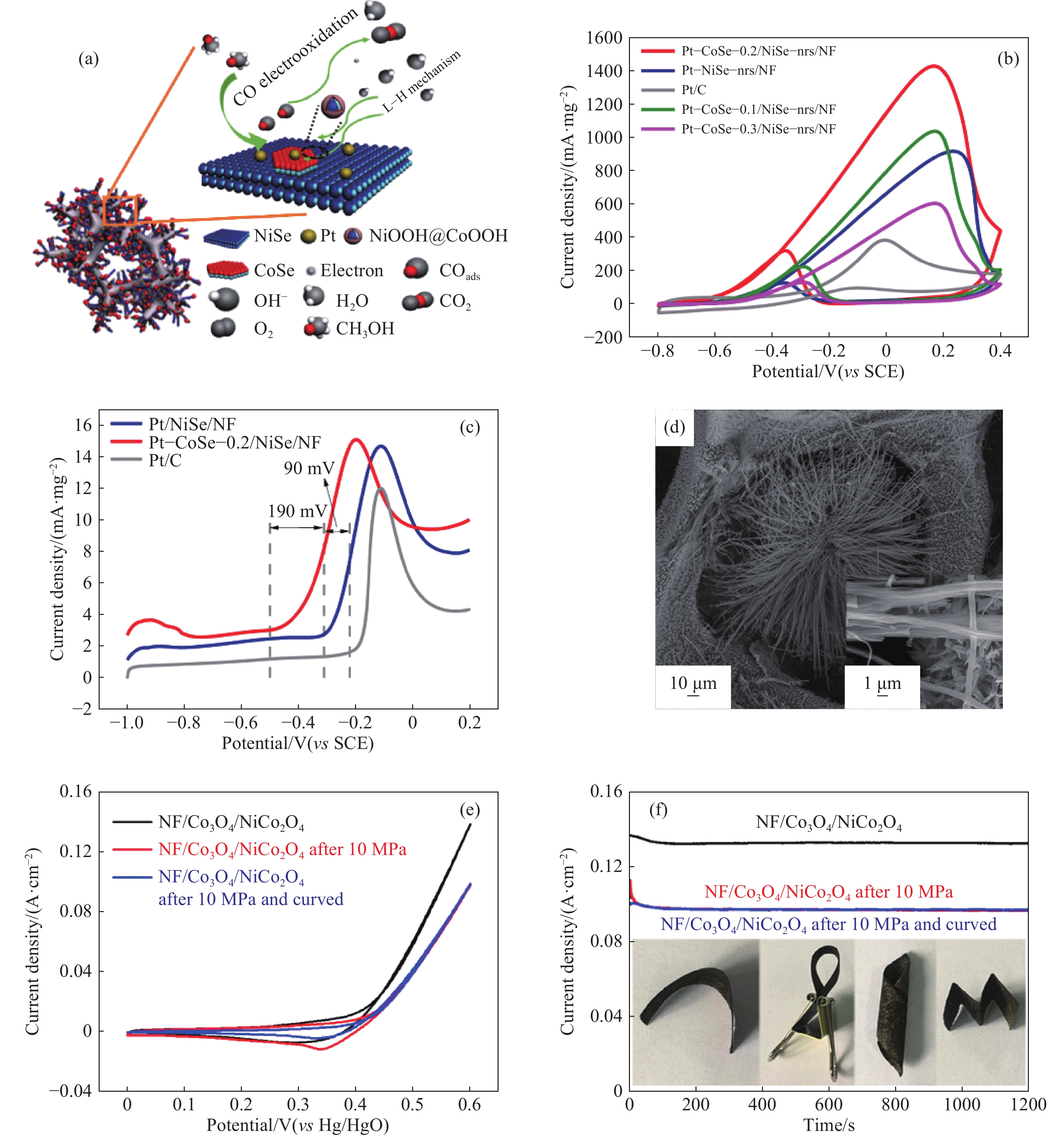
圖 8 (a)甲醇在Pt?CoSe?x/NiSe?nrs/NF上的氧化機理說明;(b)樣品在1 mol·L?1 KOH + 1 mol·L?1 CH3OH溶液中的循環伏安曲線;(c)CO剝離曲線[53];(d)NF/Co3O4/NiCo2O4的掃描電鏡圖像;(e, f)樣品在1 mol·L?1 KOH + 0.5 mol·L?1 CH3OH溶液中10 MPa壓力前后的循環伏安和計時電流曲線[54]
Figure 8. (a) Mechanism illustration of the methanol oxidation on Pt?CoSe?x/NiSe?nrs/NF; (b) CV curves of prepared samples in 1 mol·L?1 KOH + 1 mol·L?1 CH3OH; (c) CO-stripping curves[53]; (d) scanning electron microscopy image of NF/Co3O4/NiCo2O4; (e) CV and (f) CA curves of samples before and after 10 MPa in 1 mol·L?1 KOH + 0.5 mol·L?1 CH3OH[54]
表 1 不同PtM二元催化劑電催化性能的比較
Table 1. Electrocatalytic performance comparison of different PtM binary catalysts
Electrocatalysts Area activity/ (mA·cm?2) Mass activity/ (mA·mg?1) Scanning rate/
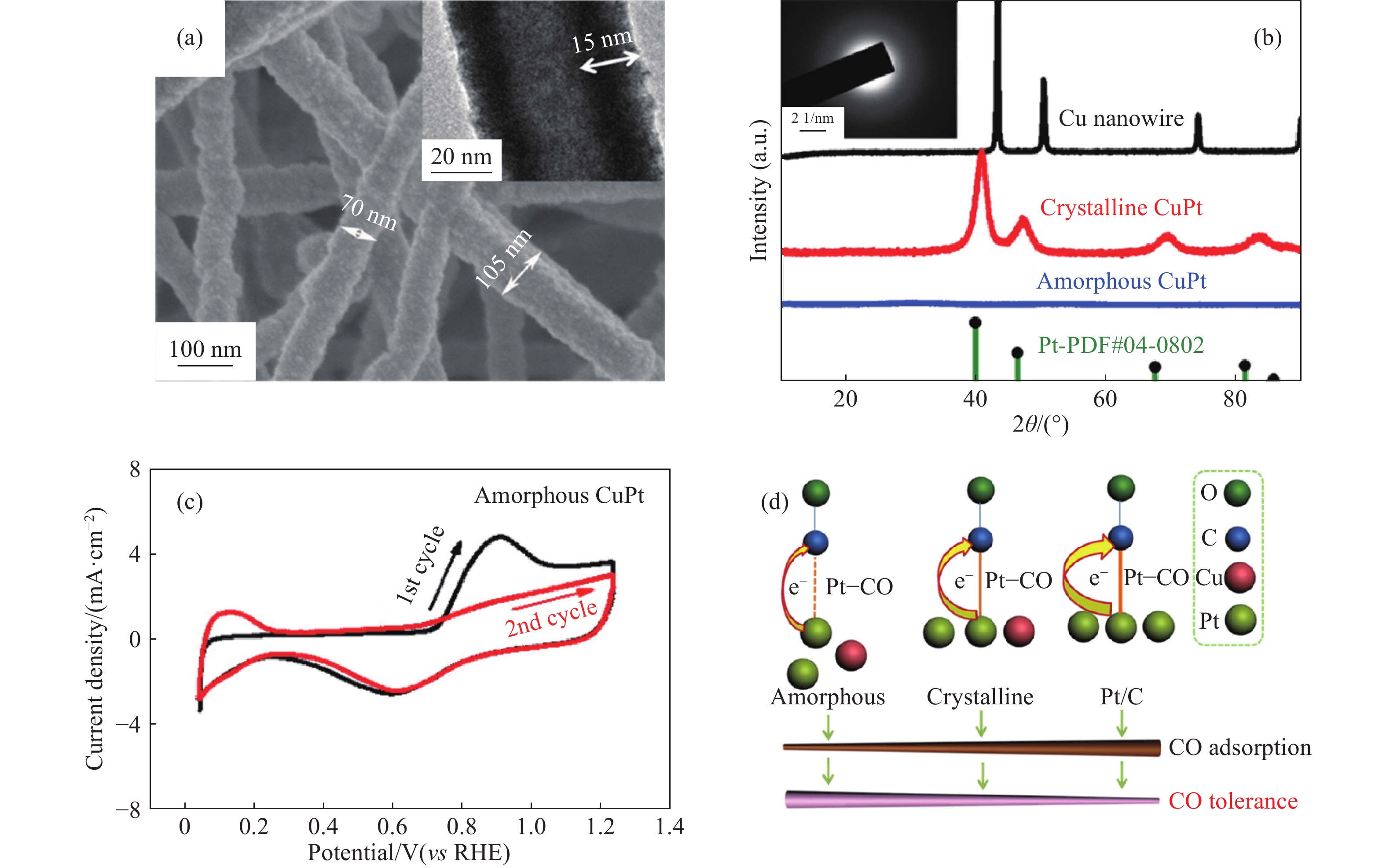
(mV·s?1)Condition Cu@CuPt[28] 5.2 2284.5 50 0.5 mol·L?1 H2SO4 + 1 mol·L?1 CH3OH PtCu[31] 34.81 NR 50 0.5 mol·L?1 H2SO4 + 1 mol·L?1 CH3OH Pt32Cu68[32] NR 707 50 0.5 mol·L?1 H2SO4 + 0.5 mol·L?1 CH3OH CuPt3[33] 2.8 634.78 50 0.5 mol·L?1 H2SO4 + 0.5 mol·L?1 CH3OH Amorphous CuPt[34] 7.8 373.7 10 0.1 mol·L?1 HClO4 + 0.5 mol·L?1 CH3OH PtCu NCs[37] 2.88 1550 50 0.1 mol·L?1 HClO4 + 1 mol·L?1 CH3OH Pt34.5Cu65.5[38] 4.12 1430 50 0.1 mol·L?1 H2SO4 + 0.5 mol·L?1 CH3OH Pt71Co29[39] 2.51 666.23 50 0.1 mol·L?1 H2SO4 + 0.5 mol·L?1 CH3OH Pt95Co5[40] 2.13 491.4 50 0.5 mol·L?1 H2SO4 + 1 mol·L?1 CH3OH Ni0.2Pt0.8[30] NR 2200 50 0.5 mol·L?1 H2SO4 + 1 mol·L?1 CH3OH Pt-Ni-P MNCs[35] 2.28 1220 50 0.5 mol·L?1 H2SO4 + 1 mol·L?1 CH3OH Note:NR is “not report”. www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Kimiaie N, Wedlich K, Hehemann M, et al. Results of a 20000 h lifetime test of a 7 kW direct methanol fuel cell (DMFC) hybrid system — degradation of the DMFC stack and the energy storage. Energy Environ Sci, 2014, 7(9): 3013 doi: 10.1039/C4EE00749B [2] Wang D Y, Chou H L, Lin Y C, et al. Simple replacement reaction for the preparation of ternary Fe1–xPtRux nanocrystals with superior catalytic activity in methanol oxidation reaction. J Am Chem Soc, 2012, 134(24): 10011 doi: 10.1021/ja3010754 [3] Zhang J M, Qu X M, Han Y, et al. Engineering PtRu bimetallic nanoparticles with adjustable alloying degree for methanol electrooxidation: Enhanced catalytic performance. Appl Catal B Environ, 2020, 263: 118345 doi: 10.1016/j.apcatb.2019.118345 [4] Tao Z C, Chen W, Yang J, et al. Ultrathin yet transferrable Pt- or PtRu-decorated graphene films as efficient electrocatalyst for methanol oxidation reaction. Sci China Mater, 2019, 62(2): 273 doi: 10.1007/s40843-018-9366-x [5] Huang L, Zhang X, Wang Q, et al. Shape-control of Pt-Ru nanocrystals: Tuning surface structure for enhanced electrocatalytic methanol oxidation. J Am Chem Soc, 2018, 140(3): 1142 doi: 10.1021/jacs.7b12353 [6] Yin S L, Kumar R D, Yu H J, et al. Pt@Mesoporous PtRu yolk-shell nanostructured electrocatalyst for methanol oxidation reaction. ACS Sustainable Chem Eng, 2019, 7(17): 14867 doi: 10.1021/acssuschemeng.9b02958 [7] Bai L. Synthesis of PtRu/Ru heterostructure for efficient methanol electrooxidation: The role of extra Ru. Appl Surf Sci, 2018, 433: 279 doi: 10.1016/j.apsusc.2017.10.026 [8] Chen F X, Ren J K, He Q, et al. Facile and one-pot synthesis of uniform PtRu nanoparticles on polydopamine-modified multiwalled carbon nanotubes for direct methanol fuel cell application. J Colloid Interface Sci, 2017, 497: 276 doi: 10.1016/j.jcis.2017.03.026 [9] Gupta D, Chakraborty S, Amorim R G, et al. Local electrocatalytic activity of PtRu supported on nitrogen-doped carbon nanotubes towards methanol oxidation by scanning electrochemical microscopy. J Mater Chem A, 2021, 9(37): 21291 doi: 10.1039/D1TA04962C [10] Wang X P, Xi S B, Lee W S V, et al. Materializing efficient methanol oxidation via electron delocalization in nickel hydroxide nanoribbon. Nat Commun, 2020, 11: 4647 doi: 10.1038/s41467-020-18459-9 [11] Zhang Z C, Luo Z M, Chen B, et al. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation. Adv Mater, 2016, 28(39): 8712 doi: 10.1002/adma.201603075 [12] Yang P P, Yuan X L, Hu H C, et al. Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation. Adv Funct Mater, 2018, 28(1): 1704774 doi: 10.1002/adfm.201704774 [13] Luo B, Zhao F, Xie Z, et al. Polyhedron-assembled ternary PtCuCo nanochains: Integrated functions enhance the electrocatalytic performance of methanol oxidation at elevated temperature. ACS Appl Mater Interfaces, 2019, 11(35): 32282 doi: 10.1021/acsami.9b10192 [14] Cui X, Xiao P, Wang J, et al. Highly branched metal alloy networks with superior activities for the methanol oxidation reaction. Angewandte Chemie, 2017, 56(16): 4488 doi: 10.1002/anie.201701149 [15] Anantharaj S, Sugime H, Noda S. Ultrafast growth of a Cu(OH)2–CuO nanoneedle array on Cu foil for methanol oxidation electrocatalysis. ACS Appl Mater Interfaces, 2020, 12(24): 27327 doi: 10.1021/acsami.0c08979 [16] Arunachalam P, Ghanem M A, Al-Mayouf A M, et al. Enhanced electrocatalytic performance of mesoporous nickel-cobalt oxide electrode for methanol oxidation in alkaline solution. Mater Lett, 2017, 196: 365 doi: 10.1016/j.matlet.2017.03.080 [17] Xue S F, Deng W T, Yang F, et al. Hexapod PtRuCu nanocrystalline alloy for highly efficient and stable methanol oxidation. ACS Catal, 2018, 8(8): 7578 doi: 10.1021/acscatal.8b00366 [18] Li H Y, Wu X S, Tao X L, et al. Direct synthesis of ultrathin Pt nanowire arrays as catalysts for methanol oxidation. Small, 2020, 16(33): 2001135 doi: 10.1002/smll.202001135 [19] Wu Y P, Tian J W, Liu S, et al. Bi-microporous metal-organic frameworks with cubane [M4(OH)4] (M=Ni, Co) clusters and pore-space partition for electrocatalytic methanol oxidation reaction. Angewandte Chemie, 2019, 131(35): 12313 doi: 10.1002/ange.201907136 [20] Long X Y, Yin P, Lei T, et al. Methanol electro-oxidation on Cu@Pt/C core-shell catalyst derived from Cu-MOF. Appl Catal B:Environ, 2020, 260: 118187 doi: 10.1016/j.apcatb.2019.118187 [21] Hamnett A. Mechanism and electrocatalysis in the direct methanol fuel cell. Catal Today, 1997, 38(4): 445 doi: 10.1016/S0920-5861(97)00054-0 [22] Tong Y Y, Yan X, Liang J, et al. Metal-based electrocatalysts for methanol electro-oxidation: Progress, opportunities, and challenges. Small, 2021, 17(9): 1904126 doi: 10.1002/smll.201904126 [23] Hsieh C T, Lin J Y. Fabrication of bimetallic Pt-M (M = Fe, Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells. J Power Sources, 2009, 188(2): 347 doi: 10.1016/j.jpowsour.2008.12.031 [24] Liu C, Chen Z L, Rao D W, et al. Behavior of gold-enhanced electrocatalytic performance of NiPtAu hollow nanocrystals for alkaline methanol oxidation. Sci China Mater, 2021, 64(3): 611 doi: 10.1007/s40843-020-1460-y [25] Guo L, Huang L B, Jiang W J, et al. Tuning the branches and composition of PtCu nanodendrites through underpotential deposition of Cu towards advanced electrocatalytic activity. J Mater Chem A, 2017, 5(19): 9014 doi: 10.1039/C7TA01859B [26] Housmans T H M, Wonders A H, Koper M T M. Structure sensitivity of methanol electrooxidation pathways on platinum: An on-line electrochemical mass spectrometry study. J Phys Chem B, 2006, 110(20): 10021 doi: 10.1021/jp055949s [27] Shenashen M A, Hassen D, El-Safty S A, et al. Axially oriented tubercle vein and X-crossed sheet of N-Co3O4@C hierarchical mesoarchitectures as potential heterogeneous catalysts for methanol oxidation reaction. Chem Eng J, 2017, 313: 83 doi: 10.1016/j.cej.2016.12.003 [28] Wang Q, Zhao Z, Jia Y, et al. Unique Cu@CuPt core-shell concave octahedron with enhanced methanol oxidation activity. ACS Appl Mater Interfaces, 2017, 9(42): 36817 doi: 10.1021/acsami.7b11268 [29] Feng Q C, Zhao S, He D S, et al. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga. J Am Chem Soc, 2018, 140(8): 2773 doi: 10.1021/jacs.7b13612 [30] Shan A X, Huang S Y, Zhao H F, et al. Atomic-scaled surface engineering Ni-Pt nanoalloys towards enhanced catalytic efficiency for methanol oxidation reaction. Nano Res, 2020, 13(11): 3088 doi: 10.1007/s12274-020-2978-3 [31] Du X W, Luo S P, Du H Y, et al. Monodisperse and self-assembled Pt-Cu nanoparticles as an efficient electrocatalyst for the methanol oxidation reaction. J Mater Chem A, 2016, 4(5): 1579 doi: 10.1039/C5TA09261B [32] Liao Y, Yu G, Zhang Y, et al. Composition-tunable PtCu alloy nanowires and electrocatalytic synergy for methanol oxidation reaction. J Phys Chem C, 2016, 120(19): 10476 doi: 10.1021/acs.jpcc.6b02630 [33] Fu G, Yan X, Cui Z, et al. Catalytic activities for methanol oxidation on ultrathin CuPt3 wavy nanowires with/without smart polymer. Chem Sci, 2016, 7(8): 5414 doi: 10.1039/C6SC01501H [34] Zhao Y G, Liu J J, Liu C G, et al. Amorphous CuPt alloy nanotubes induced by Na2S2O3 as efficient catalysts for the methanol oxidation reaction. ACS Catal, 2016, 6(7): 4127 doi: 10.1021/acscatal.6b00540 [35] Deng K, Xu Y, Yang D D, et al. Pt–Ni–P nanocages with surface porosity as efficient bifunctional electrocatalysts for oxygen reduction and methanol oxidation. J Mater Chem A, 2019, 7(16): 9791 doi: 10.1039/C9TA00928K [36] Yin S L, Wang Z Q, Li C J, et al. Mesoporous Pt@PtM (M = Co, Ni) cage-bell nanostructures toward methanol electro-oxidation. Nanoscale Adv, 2020, 2(3): 1084 doi: 10.1039/D0NA00020E [37] Eid K, Wang H, He P, et al. One-step synthesis of porous bimetallic PtCu nanocrystals with high electrocatalytic activity for methanol oxidation reaction. Nanoscale, 2015, 7(40): 16860 doi: 10.1039/C5NR04557F [38] Li C, Liu T, He T, et al. Composition-driven shape evolution to Cu-rich PtCu octahedral alloy nanocrystals as superior bifunctional catalysts for methanol oxidation and oxygen reduction reaction. Nanoscale, 2018, 10(10): 4670 doi: 10.1039/C7NR09669K [39] Zhang L, Zhang X F, Chen X L, et al. Facile solvothermal synthesis of Pt71Co29 lamellar nanoflowers as an efficient catalyst for oxygen reduction and methanol oxidation reactions. J Colloid Interface Sci, 2019, 536: 556 doi: 10.1016/j.jcis.2018.10.080 [40] Lu Q Q, Sun L T, Zhao X, et al. One-pot synthesis of interconnected Pt95Co5 nanowires with enhanced electrocatalytic performance for methanol oxidation reaction. Nano Res, 2018, 11(5): 2562 doi: 10.1007/s12274-017-1881-z [41] Kwon T, Jun M, Kim H Y, et al. Vertex-reinforced PtCuCo ternary nanoframes as efficient and stable electrocatalysts for the oxygen reduction reaction and the methanol oxidation reaction. Adv Funct Mater, 2018, 28(13): 1706440 doi: 10.1002/adfm.201706440 [42] Zhang P F, Dai X P, Zhang X, et al. One-pot synthesis of ternary Pt–Ni–Cu nanocrystals with high catalytic performance. Chem Mater, 2015, 27(18): 6402 doi: 10.1021/acs.chemmater.5b02575 [43] Sun Y, Zhou Y J, Zhu C, et al. Synergistic Cu@CoOx core-cage structure on carbon layers as highly active and durable electrocatalysts for methanol oxidation. Appl Catal B:Environ, 2019, 244: 795 doi: 10.1016/j.apcatb.2018.12.017 [44] Ghouri Z K, Barakat N A M, Kim H Y. Influence of copper content on the electrocatalytic activity toward methanol oxidation of CoχCuy alloy nanoparticles-decorated CNFs. Sci Rep, 2015, 5: 16695 doi: 10.1038/srep16695 [45] Wu D F, Zhang W, Cheng D J. Facile synthesis of Cu/NiCu electrocatalysts integrating alloy, core–shell, and one-dimensional structures for efficient methanol oxidation reaction. ACS Appl Mater Interfaces, 2017, 9(23): 19843 doi: 10.1021/acsami.7b03876 [46] Kaur B, Anu Prathap M U, Srivastava R. Synthesis of transition-metal exchanged nanocrystalline ZSM-5 and their application in electrochemical oxidation of glucose and methanol. ChemPlusChem, 2012, 77(12): 1119 doi: 10.1002/cplu.201200236 [47] Kaur B, Srivastava R, Satpati B. Highly efficient CeO2 decorated nano-ZSM-5 catalyst for electrochemical oxidation of methanol. ACS Catal, 2016, 6(4): 2654 doi: 10.1021/acscatal.6b00525 [48] Samanta S, Bhunia K, Pradhan D, et al. Ni and Cu ion-exchanged nanostructured mesoporous zeolite: A noble metal free, efficient, and durable electrocatalyst for alkaline methanol oxidation reaction. Mater Today Energy, 2018, 8: 45 doi: 10.1016/j.mtener.2018.02.007 [49] Samanta S, Bhunia K, Pradhan D, et al. NiCuCo2O4 supported Ni-Cu ion-exchanged mesoporous zeolite heteronano architecture: An efficient, stable, and economical nonprecious electrocatalyst for methanol oxidation. ACS Sustain Chem Eng, 2018, 6(2): 2023 doi: 10.1021/acssuschemeng.7b03444 [50] Lu X F, Xia B Y, Zang S Q, et al. Metal-organic frameworks based electrocatalysts for the oxygen reduction reaction. Angewandte Chemie Int Ed, 2020, 59(12): 4634 doi: 10.1002/anie.201910309 [51] Zhang X, Chen A, Zhong M, et al. Metal-organic frameworks (MOFs) and MOF-derived materials for energy storage and conversion. Electrochem Energy Rev, 2019, 2(1): 29 doi: 10.1007/s41918-018-0024-x [52] Tang C Y, Asefa T. Ternary ZIF-8-derived dual-metal CoCu nanoparticles in porous carbon polyhedra as efficient catalysts for methanol oxidation. J Mater Chem A, 2020, 8(25): 12285 doi: 10.1039/D0TA04146G [53] Du J N, You S J, Li X R, et al. In situ crystallization of active NiOOH/CoOOH heterostructures with hydroxide ion adsorption sites on velutipes-like CoSe/NiSe nanorods as catalysts for oxygen evolution and cocatalysts for methanol oxidation. ACS Appl Mater Interfaces, 2020, 12(1): 686 doi: 10.1021/acsami.9b16626 [54] Qian L, Luo S L, Wu L S, et al. In situ growth of metal organic frameworks derived hierarchical hollow porous Co3O4/NiCo2O4 nanocomposites on nickel foam as self-supported flexible electrode for methanol electrocatalytic oxidation. Appl Surf Sci, 2020, 503: 144306 doi: 10.1016/j.apsusc.2019.144306 -




 下載:
下載: