Preparation of nanosized red phosphorus and its application in sodium-ion batteries
-
摘要: 鈉離子電池(SIBs)具有成本低廉、安全性高、環境友好等優點,且可以兼容現有的鋰離子電池生產設備,在大規模儲能以及電動汽車領域都有著廣泛的應用前景。在眾多的SIBs負極材料中,紅磷擁有超高的理論比容量(2596 mA·h·g–1)、合適的氧化還原電位(0.4 V vs Na/Na+)以及豐富的資源儲量,是極具潛力的SIBs負極材料。然而紅磷極低的本征電導率和在儲鈉過程中巨大的體積效應極大的限制了其容量利用率、長期循環穩定性和倍率性能。目前對紅磷基負極材料改性的最有效方法之一是紅磷的納米化,納米化可以改善紅磷的電化學活性和長期循環穩定性。為了便于研究者了解納米紅磷的制備方法,本文系統總結了納米紅磷的制備方法,包括球磨、升華冷凝、熱還原、氣相生長、溶劑熱、化學沉淀等,并對各種方法的優缺點進行了分析比較,最后對未來的研究方向進行了展望。希望能以此促進紅磷負極的發展及其在鈉離子電池中的實際應用。Abstract: Sodium-ion batteries (SIBs) are highly desirable energy storage devices because of their low cost, high safety, and environmental compatibility. Therefore, SIBs have wide application prospects in the fields of large-scale energy storage and electric vehicles. SIBs have a similar energy storage mechanism as that of lithium-ion batteries (LIBs) and can be fabricated using existing LIB production equipment. Thus, SIBs are the most promising alternative to LIBs. However, the radius of Na+ is ~34% larger than that of Li+; therefore, many electrode materials developed for LIBs are unsuitable for SIBs. The exploration of novel electrode materials for SIBs has garnered significant interest in recent years. Among various candidate anode materials for SIBs, red phosphorus is a promising material owing to its ultrahigh theoretical specific capacity (2596 mA·h·g–1), suitable oxidation–reduction potential (0.4 V vs Na/Na+), and abundance. However, the capacity utilization, long-term cycle stability, and rate performance of red phosphorous are limited due to its low intrinsic conductivity and a large volume effect upon sodium storage. At present, an effective approach for the modification of red phosphorus anodes is to prepare nanosized red phosphorus (NRP). Miniaturizing red phosphorus prevents structural damage via large volume changes during charge/discharge processes and also shortens Na+ transmission distances, which enables high electrochemical activity and long-term cycling stability. Herein, recent studies on NRP preparation for advanced SIBs are extensively reviewed. NRP preparation methods typically include ball milling, vaporization condensation, and chemical deposition. Other novel approaches such as thermal reduction, vapor growth, and solvothermal synthesis have also been reported. Ball milling is straightforward and scalable; however, strict guidelines are required to prevent the red phosphorus from burning and exploding, and slight oxidation and particle aggregation are unavoidable. Vaporization-condensation strategies are suitable for the uniform deposition of NRP onto a matrix but are limited by low phosphorus loading and residual white phosphorus. Chemical deposition methods are promising due to their simplicity, control over particle size, and scalability. There are two main chemical deposition strategies, i.e., the reduction of phosphorus-containing compounds and the dissolution and deposition of phosphorus amines. The former method is facile and compatible with ambient temperatures, while the latter method is safe, cost effective, and has high yields. Further studies should focus on morphology design, increasing phosphorus loading, and developing novel chemical reduction methods. We hope that this review promotes the development of red phosphorous anodes for application in SIBs.
-
Key words:
- batteries /
- sodium ion /
- anodes /
- red phosphorus /
- nano-size
-
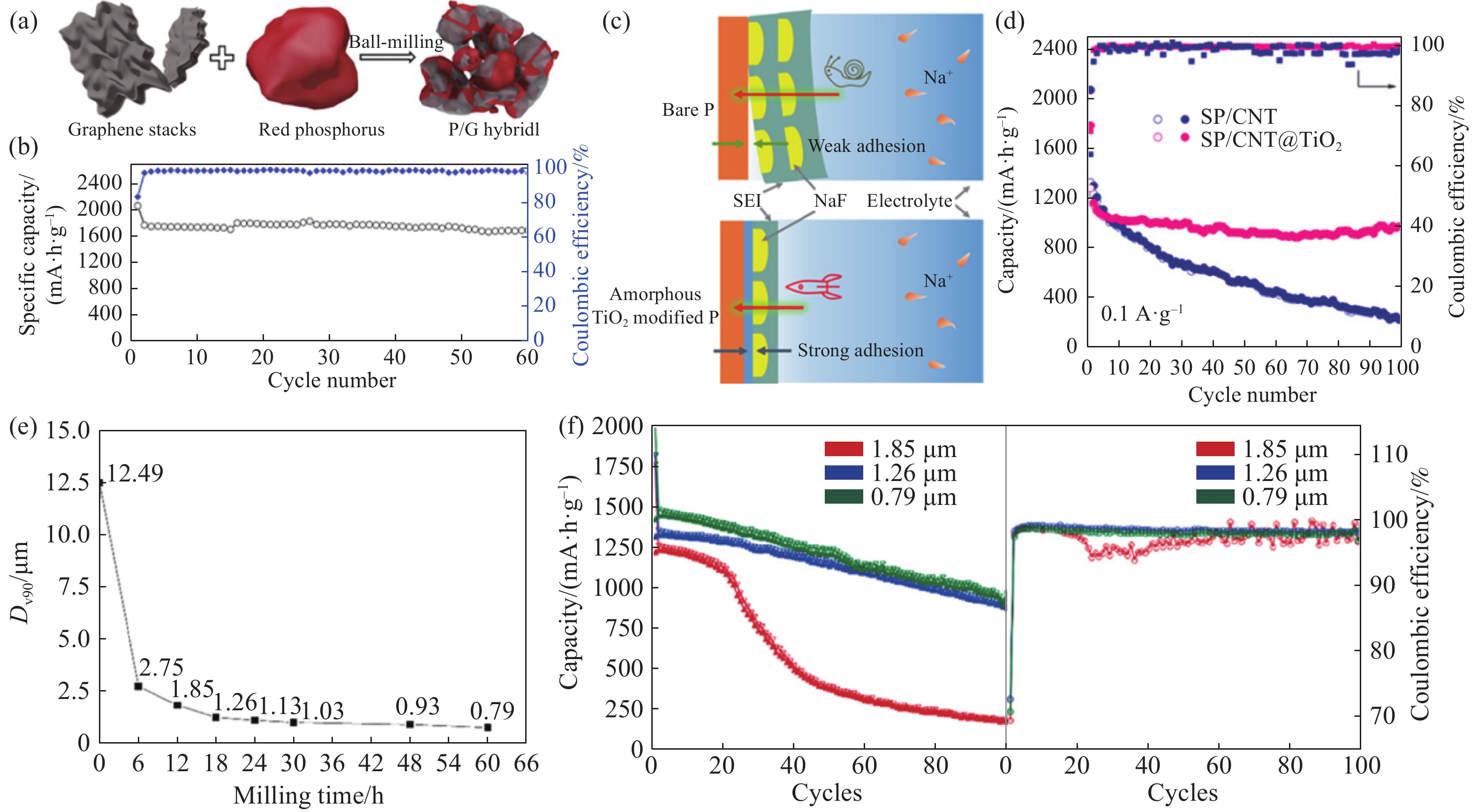
圖 1 (a)P/G復合材料制備示意圖[18];(b)P/G復合材料在260 mA·g–1下的循環性能[18];(c)SEI組分對鈉離子存儲性能的影響[29];(d)SP/CNT和SP/CNT@TiO2的循環性能[29];(e)Dv90隨球磨時間的變化曲線[30];(f)不同Dv90的球磨紅磷的循環性能和庫侖效率[30]
Figure 1. (a) Schematic of synthesis of P/G hybrid[18]; (b) cycling performance of P/G hybrid anode at a current density of 260 mA·g–1[18]; (c) illustration of different components of the SEI film and their contribution to sodium storage in different electrodes[29]; (d) cycling performance of SP/CNT and SP/CNT@TiO2[29]; (e) change curve of Dv90 with ball milling time[30]; (f) cycling performance and Coulombic efficiencies of ball-milled red phosphorus with different Dv90[30]
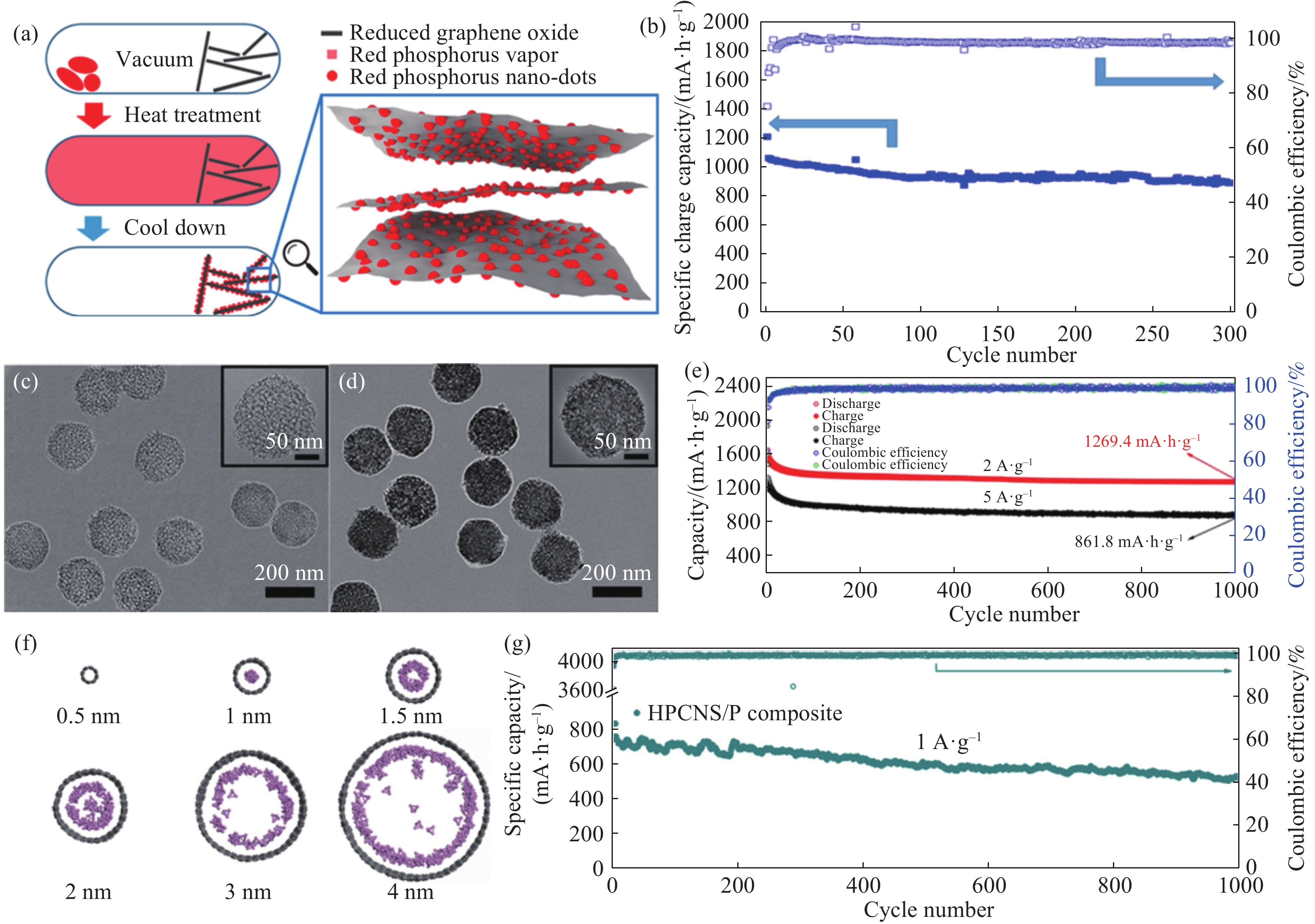
圖 2 (a)P@rGO的制備示意圖[35];(b)P@rGO在1593.9 mA·g–1下的循環性能[35];(c, d) HHPCNSs和HHPCNSs/P的TEM照片[45];(e)HHPCNSs/P在2 A·g–1和5 A·g–1下的循環性能[45];(f)P4吸附到直徑為 0.5、1、1.5、2、3和4 nm的納米管后平衡形態頂視圖[43];(g)HPCNS/P在1 A·g–1下的長期循環性能圖[43]
Figure 2. (a) Schematic of P@rGO synthesis[35]; (b) cycling performance of P@rGO at a current density of 1593.9 mA·g?1[35]; (c, d) TEM image of HHPCNSs and HHPCNSs/P[45]; (e) cycling performance of P@rGO at a current density of 2 and 5 A·g–1 [45]; (f) top view of the equilibrium morphologies after P4 adsorption into the nanotubes of 0.5, 1, 1.5, 2, 3, and 4-nm diameter[43]; (g) long-term cycle stability of the HPCNS/P composite electrode for 1000 cycles at 1.0 A·g?1[43]
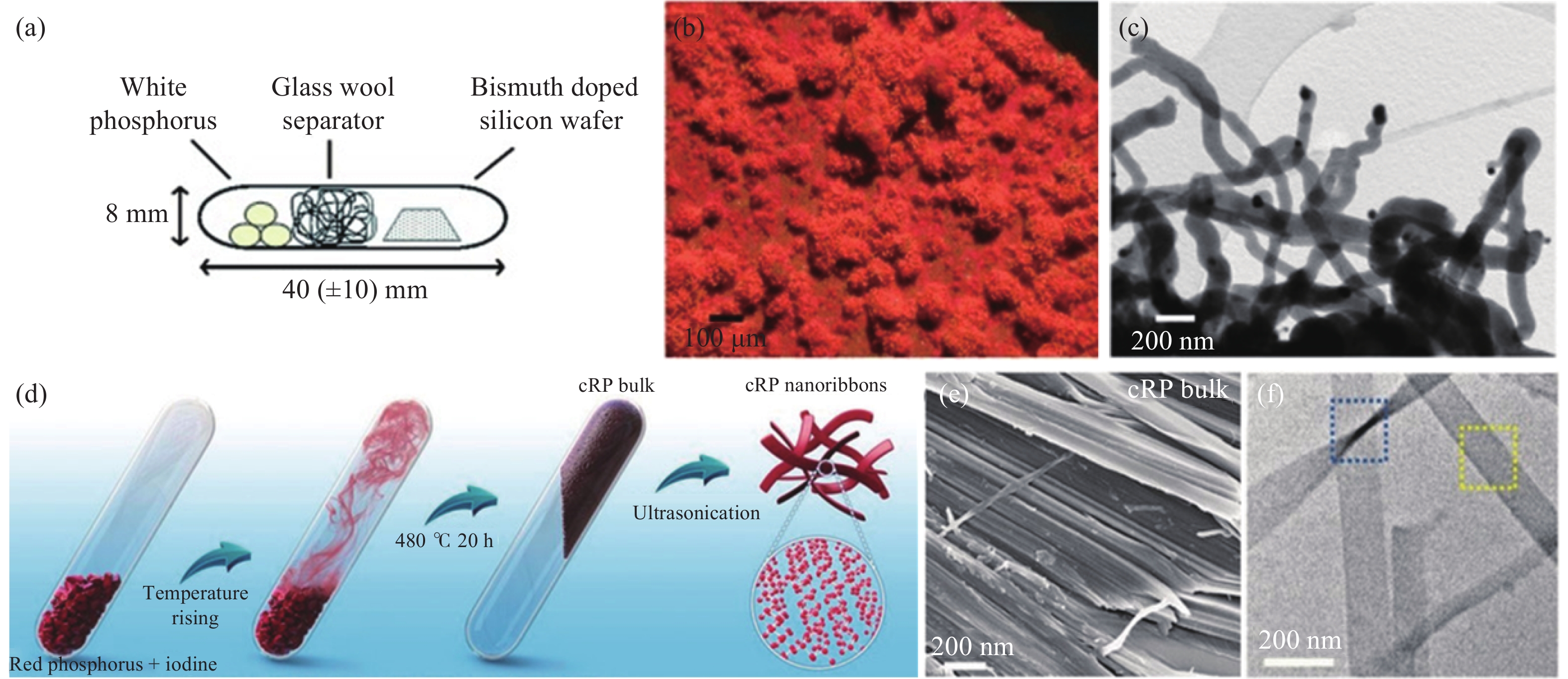
圖 4 (a)晶體紅磷納米線的制備示意圖[58];(b)熱處理后的光學照片[58];(c)晶體紅磷納米線的TEM照片[58];(d)塊狀晶態紅磷和晶體紅磷納米帶的制備示意圖[60];(e)塊狀晶體紅磷的SEM照片[60];(f)晶體紅磷納米帶的TEM照片[60]
Figure 4. (a) Schematic of the synthesis of crystalline red phosphorus nanowire[58]; (b) optical micrographs of the product after heat treatment[58]; (c) TEM image of the crystalline red phosphorus nanowire[58]; (d) schematic of the synthesis of red phosphorus bulk and nanoribbons[60]; (e) SEM image of red phosphorus bulk[60]; (f) TEM image of red phosphorus nanoribbons[60]
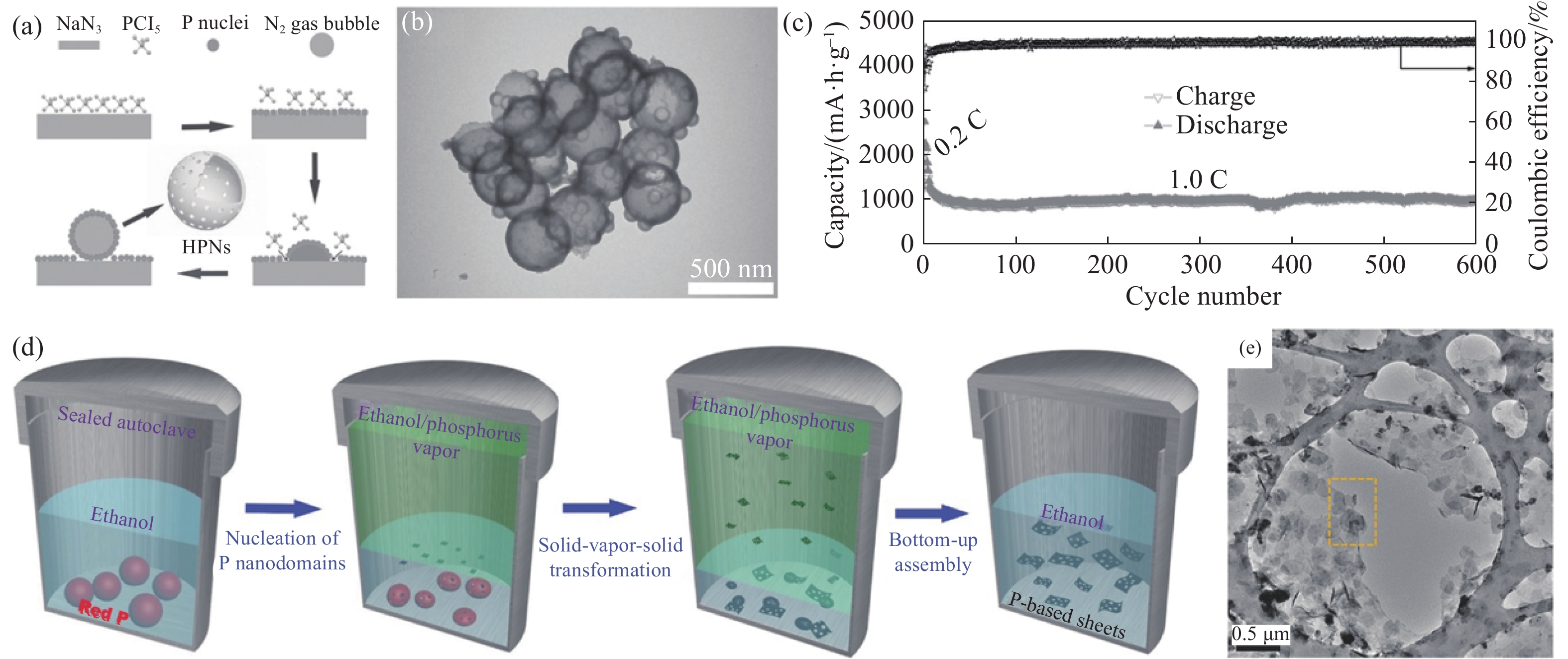
圖 5 (a)HPNs的生成機理圖[61];(b)HPNs的TEM照片[61];(c)HPNs在2.6 A·g?1下的循環性能圖[61] ;(d)磷納米片的生成機理圖[62];(e)磷納米片的TEM照片[62]
Figure 5. (a) Schematic of the formation mechanism of HPNs[61]; (b) TEM image of HPNs[61]; (c) cycling performance of HPNs at a current density of 2.6 A·g?1[61]; (d) schematic of the formation of phosphorus nanosheets[62]; (e)TEM image of phosphorus nanosheets[62]
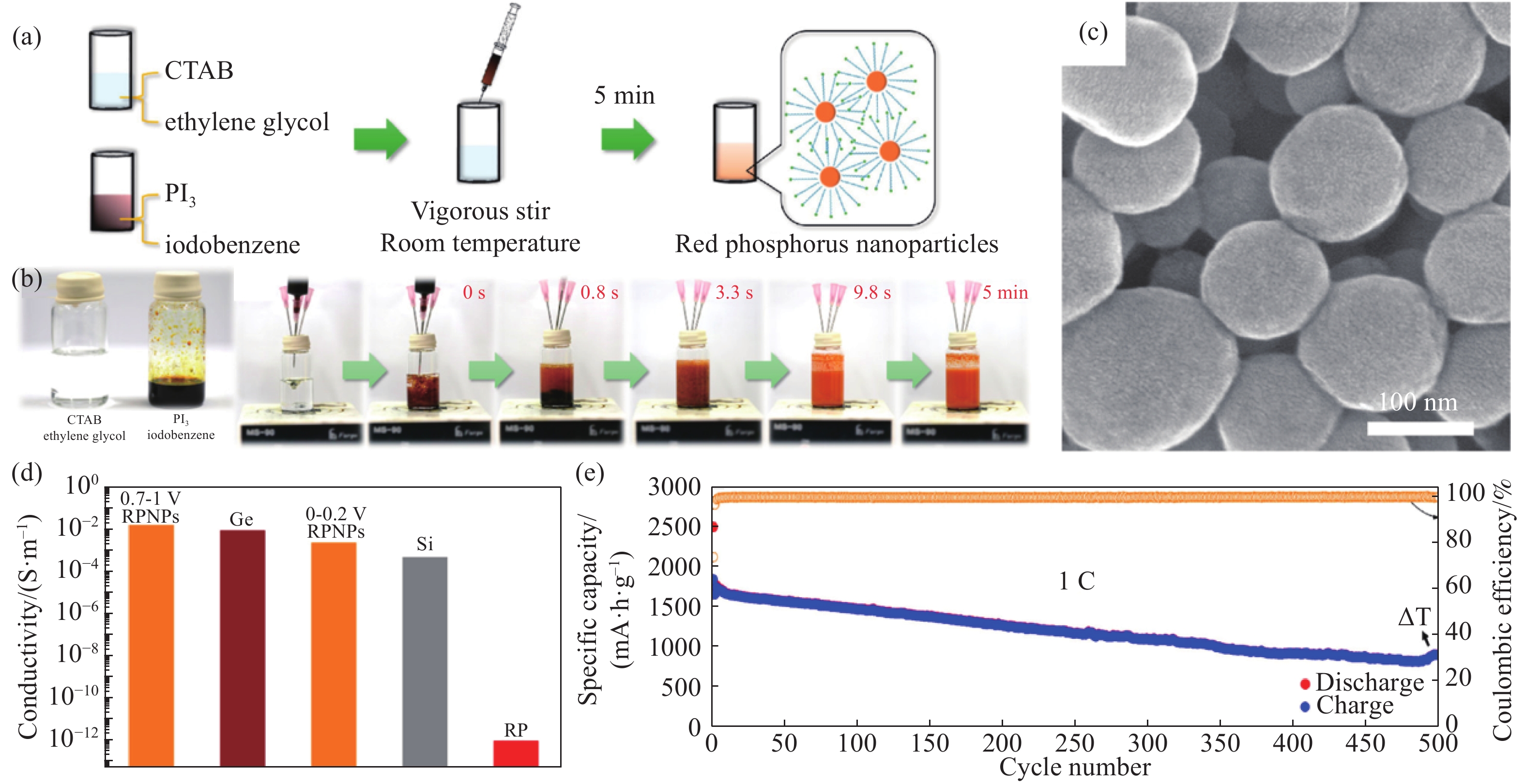
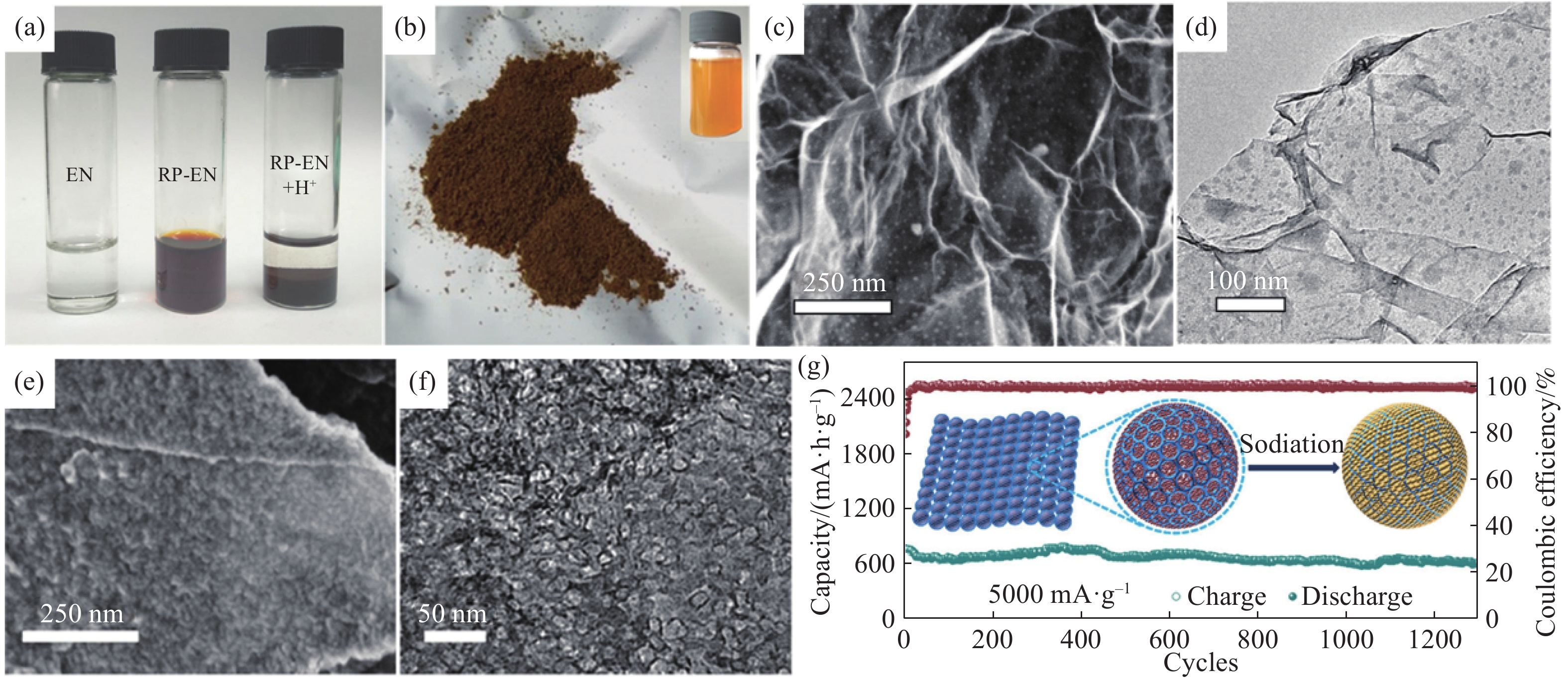
圖 7 (a)EN溶液、RP?EN溶液、RP?EN溶液混合稀HCl的光學照片[72];(b)NRP粉體和NRP膠體的光學照片[72];(c)NRP?rGO的SEM照片[72];(d)NRP?rGO的TEM照片[72];(e, f)RP@CNC的SEM和TEM照片[73];(g)RP@CNCs在5 A·g?1下的循環性能圖[73]
Figure 7. (a) Photographs of the EN solution, RP?EN complex solution, and RP?EN complex mixed with dilute hydrochloric acid[72]; (b) photographs of the NRP powder and NRP colloid[72]; (c) SEM image of NRP?rGO; (d) TEM of NRP?rGO[72]; (e, f) SEM and TEM images of RP@CNC[73]; (g) cycling performance of RP@CNC at a current density of 5 A·g?1[73]
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Hwang J Y, Myung S T, Aurbach D, et al. Effect of nickel and iron on structural and electrochemical properties of O3 type layer cathode materials for sodium-ion batteries. J Power Sources, 2016, 324: 106 doi: 10.1016/j.jpowsour.2016.05.064 [2] Wang Y S, Mu L Q, Liu J, et al. A novel high capacity positive electrode material with tunnel-type structure for aqueous sodium-ion batteries. Adv Energy Mater, 2015, 5(22): 1501005 doi: 10.1002/aenm.201501005 [3] Jian Z L, Zhao L, Pan H L, et al. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem Commun, 2012, 14(1): 86 doi: 10.1016/j.elecom.2011.11.009 [4] Wang W L, Gang Y, Hu Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat Commun, 2020, 11: 980 doi: 10.1038/s41467-020-14444-4 [5] Li Y Q, Lu Y X, Meng Q S, et al. Regulating pore structure of hierarchical porous waste cork-derived hard carbon anode for enhanced Na storage performance. Adv Energy Mater, 2019, 9(48): 1902852 doi: 10.1002/aenm.201902852 [6] Kong D Z, Wang Y, Huang S Z, et al. Surface modification of Na2Ti3O7 nanofibre arrays using N-doped graphene quantum dots as advanced anodes for sodium-ion batteries with ultra-stable and high-rate capability. J Mater Chem A, 2019, 7(20): 12751 doi: 10.1039/C9TA01641D [7] Liu J, Wen Y R, van Aken P A, et al. Facile synthesis of highly porous Ni–Sn intermetallic microcages with excellent electrochemical performance for lithium and sodium storage. Nano Lett, 2014, 14(11): 6387 doi: 10.1021/nl5028606 [8] Sottmann J, Herrmann M, Vajeeston P, et al. How crystallite size controls the reaction path in nonaqueous metal ion batteries: The example of sodium bismuth alloying. Chem Mater, 2016, 28(8): 2750 doi: 10.1021/acs.chemmater.6b00491 [9] Xie X Q, Makaryan T, Zhao M Q, et al. MoS2 nanosheets vertically aligned on carbon paper: A freestanding electrode for highly reversible sodium-ion batteries. Adv Energy Mater, 2016, 6(5): 1502161 doi: 10.1002/aenm.201502161 [10] Liu S H, Wang Y W, Dong Y F, et al. Ultrafine Fe3O4 quantum dots on hybrid carbon nanosheets for long-life, high-rate alkali-metal storage. ChemElectroChem, 2016, 3(1): 38 doi: 10.1002/celc.201500410 [11] Shi S S, Sun C L, Yin X P, et al. FeP quantum dots confined in carbon-nanotube-grafted P-doped carbon octahedra for high-rate sodium storage and full-cell applications. Adv Funct Mater, 2020, 30(10): 1909283 doi: 10.1002/adfm.201909283 [12] Qian J F, Wu X Y, Cao Y L, et al. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angewandte Chemie Int Ed, 2013, 52(17): 4633 doi: 10.1002/anie.201209689 [13] Zhao R Z, Qian Z, Liu Z Y, et al. Molecular-level heterostructures assembled from layered black phosphorene and Ti3C2 MXene as superior anodes for high-performance sodium ion batteries. Nano Energy, 2019, 65: 104037 doi: 10.1016/j.nanoen.2019.104037 [14] Lan D N, Li Q. Manipulating local chemistry of phosphorus for high-performance sodium ion battery anode applications. ACS Appl Energy Mater, 2019, 2(1): 661 doi: 10.1021/acsaem.8b01666 [15] Song J X, Yu Z X, Gordin M L, et al. Advanced sodium ion battery anode constructed via chemical bonding between phosphorus, carbon nanotube, and cross-linked polymer binder. ACS Nano, 2015, 9(12): 11933 doi: 10.1021/acsnano.5b04474 [16] Zhang Z J, Li W J, Chou S L, et al. Effects of carbon on electrochemical performance of red phosphorus (P) and carbon composite as anode for sodium ion batteries. J Mater Sci Technol, 2021, 68: 140 doi: 10.1016/j.jmst.2020.08.034 [17] Li W J, Chou S L, Wang J Z, et al. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett, 2013, 13(11): 5480 doi: 10.1021/nl403053v [18] Song J, Yu Z, Gordin M L, et al. Chemically bonded phosphorus/graphene hybrid as a high performance anode for sodium-ion batteries. Nano Lett, 2014, 14(11): 6329 doi: 10.1021/nl502759z [19] Feng N, Liang X Q, Pu X, et al. Rational design of red phosphorus/reduced graphene oxide composites for stable sodium ion storage. J Alloys Compd, 2019, 775: 1270 doi: 10.1016/j.jallcom.2018.10.143 [20] Li W J, Han C, Gu Q F, et al. Three-dimensional electronic network assisted by TiN conductive Pillars and chemical adsorption to boost the electrochemical performance of red phosphorus. ACS Nano, 2020, 14(4): 4609 doi: 10.1021/acsnano.0c00216 [21] Li W J, Chou S L, Wang J Z, et al. Significant enhancement of the cycling performance and rate capability of the P/C composite via chemical bonding (P–C). J Mater Chem A, 2016, 4(2): 505 doi: 10.1039/C5TA08590J [22] Zhang Y K, Tao H C, Li J H, et al. Achieving a high-performance P/C anode through P–O–C bond for sodium ion batteries. Ionics, 2020, 26(7): 3377 doi: 10.1007/s11581-020-03486-9 [23] Hu Y, Li B, Jiao X X, et al. Stable cycling of phosphorus anode for sodium-ion batteries through chemical bonding with sulfurized polyacrylonitrile. Adv Funct Mater, 2018, 28(23): 1801010 doi: 10.1002/adfm.201801010 [24] Wang Y T, Yang X D, Zhao C Y, et al. Improving the structure stabilization of red phosphorus anodes via the shape memory effect of a Ni–Ti alloy for high-performance sodium ion batteries. Chem Commun, 2019, 55(32): 4659 doi: 10.1039/C9CC00024K [25] Walter M, Erni R, Kovalenko M V. Inexpensive antimony nanocrystals and their composites with red phosphorus as high-performance anode materials for Na-ion batteries. Sci Rep, 2015, 5: 8418 doi: 10.1038/srep08418 [26] Chin L C, Yi Y H, Chang W C, et al. Significantly improved performance of red phosphorus sodium-ion anodes with the addition of iron. Electrochimica Acta, 2018, 266: 178 doi: 10.1016/j.electacta.2017.12.105 [27] Liu Y, Ru Q, Gao Y Q, et al. Synthesis and electrochemical research of milled antimony and red phosphorus hybrid inlaid with graphene sheets as anodes for lithium-sodium storage. Energy Technol, 2019, 7(6): 1801022 doi: 10.1002/ente.201801022 [28] Mi H W, Wang Y T, Chen H, et al. Boosting Na-ion diffusion by piezoelectric effect induced by alloying reaction of micro red-phosphorus/BaTiO3/graphene composite anode. Nano Energy, 2019, 66: 104136 doi: 10.1016/j.nanoen.2019.104136 [29] Zhang J, Zhang K, Yang J, et al. Engineering solid electrolyte interphase on red phosphorus for long-term and high-capacity sodium storage. Chem Mater, 2020, 32(1): 448 doi: 10.1021/acs.chemmater.9b04043 [30] Capone I, Hurlbutt K, Naylor A J, et al. Effect of the particle-size distribution on the electrochemical performance of a red phosphorus-carbon composite anode for sodium-ion batteries. Energy Fuels, 2019, 33(5): 4651 doi: 10.1021/acs.energyfuels.9b00385 [31] Fang K, Liu D, Xiang X Y, et al. Air-stable red phosphorus anode for potassium/sodium-ion batteries enabled through dual-protection design. Nano Energy, 2020, 69: 104451 doi: 10.1016/j.nanoen.2020.104451 [32] Yu Z X, Song J X, Wang D W, et al. Advanced anode for sodium-ion battery with promising long cycling stability achieved by tuning phosphorus-carbon nanostructures. Nano Energy, 2017, 40: 550 doi: 10.1016/j.nanoen.2017.08.019 [33] Tian W F, Wang L, Huo K F, et al. Red phosphorus filled biomass carbon as high-capacity and long-life anode for sodium-ion batteries. J Power Sources, 2019, 430: 60 doi: 10.1016/j.jpowsour.2019.04.086 [34] Zhou J B, Liu X J, Zhu L Q, et al. High-spin sulfur-mediated phosphorous activation enables safe and fast phosphorus anodes for sodium-ion batteries. Chem, 2020, 6(1): 221 doi: 10.1016/j.chempr.2019.10.021 [35] Liu Y, Zhang A, Shen C, et al. Red phosphorus nanodots on reduced graphene oxide as a flexible and ultra-fast anode for sodium-ion batteries. ACS Nano, 2017, 11(6): 5530 doi: 10.1021/acsnano.7b00557 [36] Zhu Y J, Wen Y, Fan X L, et al. Red phosphorus-single-walled carbon nanotube composite as a superior anode for sodium ion batteries. ACS Nano, 2015, 9(3): 3254 doi: 10.1021/acsnano.5b00376 [37] Wu N, Yao H R, Yin Y X, et al. Improving the electrochemical properties of the red P anode in Na-ion batteries via the space confinement of carbon nanopores. J Mater Chem A, 2015, 3(48): 24221 doi: 10.1039/C5TA08367B [38] Ruan J, Mo F, Long Z, et al. Tailor-made gives the best fits: Superior Na/K-ion storage performance in exclusively confined red phosphorus system. ACS Nano, 2020, 14(9): 12222 doi: 10.1021/acsnano.0c05951 [39] Liu Y H, Liu Q Z, Jian C, et al. Red-phosphorus-impregnated carbon nanofibers for sodium-ion batteries and liquefaction of red phosphorus. Nat Commun, 2020, 11: 2520 doi: 10.1038/s41467-020-16077-z [40] Wu Y, Xing F F, Xu R, et al. Spatially confining and chemically bonding amorphous red phosphorus in the nitrogen doped porous carbon tubes leading to superior sodium storage performance. J Mater Chem A, 2019, 7(14): 8581 doi: 10.1039/C9TA01039D [41] Sun X Z, Li W H, Zhong X W, et al. Superior sodium storage in phosphorus@porous multichannel flexible freestanding carbon nanofibers. Energy Storage Mater, 2017, 9: 112 doi: 10.1016/j.ensm.2017.07.003 [42] Ruan B Y, Wang J, Shi D Q, et al. A phosphorus/N-doped carbon nanofiber composite as an anode material for sodium-ion batteries. J Mater Chem A, 2015, 3(37): 19011 doi: 10.1039/C5TA04366B [43] Yao S S, Cui J, Huang J Q, et al. Rational assembly of hollow microporous carbon spheres as P hosts for long-life sodium-ion batteries. Adv Energy Mater, 2018, 8(7): 1702267 doi: 10.1002/aenm.201702267 [44] Jin H L, Lu H, Wu W Y, et al. Tailoring conductive networks within hollow carbon nanospheres to host phosphorus for advanced sodium ion batteries. Nano Energy, 2020, 70: 104569 doi: 10.1016/j.nanoen.2020.104569 [45] Liu B, Zhang Q, Li L, et al. Encapsulating red phosphorus in ultralarge pore volume hierarchical porous carbon nanospheres for lithium/sodium-ion half/full batteries. ACS Nano, 2019, 13(11): 13513 doi: 10.1021/acsnano.9b07428 [46] Xu J, Ding J N, Zhu W J, et al. Nano-structured red phosphorus/porous carbon as a superior anode for lithium and sodium-ion batteries. Sci China Mater, 2018, 61(3): 371 doi: 10.1007/s40843-017-9152-9 [47] Han L F, Wang J L, Mu X W, et al. Anisotropic, low-tortuosity and ultra-thick red P@C-Wood electrodes for sodium-ion batteries. Nanoscale, 2020, 12(27): 14642 doi: 10.1039/D0NR03059G [48] Zhao D, Li B B, Zhang J Y, et al. A hierarchical phosphorus nanobarbed nanowire hybrid: Its structure and electrochemical properties. Nano Lett, 2017, 17(6): 3376 doi: 10.1021/acs.nanolett.6b05301 [49] Qin G, Duan J, Yang Y, et al. Magnetic field facilitated resilient chain-like Fe3O4/C/red P with superior sodium storage performance. ACS Appl Mater Interfaces, 2018, 10(7): 6441 doi: 10.1021/acsami.7b17341 [50] Cheng J, Zhang G, Wang P, et al. Confined red phosphorus in edible fungus slag-derived porous carbon as an improved anode material in sodium-ion batteries. ACS Appl Mater Interfaces, 2019, 11(51): 47948 doi: 10.1021/acsami.9b17123 [51] Sun J, Lee H W, Pasta M, et al. Carbothermic reduction synthesis of red phosphorus-filled 3D carbon material as a high-capacity anode for sodium ion batteries. Energy Storage Mater, 2016, 4: 130 doi: 10.1016/j.ensm.2016.04.003 [52] Li T Q, Lin N, Han Y, et al. Metallothermic reduction of molten adduct [PCl4+][AlCl4?] at 50 ℃ to amorphous phosphorus or crystallized phosphides. ACS Appl Mater Interfaces, 2018, 10(49): 42469 doi: 10.1021/acsami.8b16481 [53] Zhu L, Zhu Z, Zhou J, et al. Kirkendall effect modulated hollow red phosphorus nanospheres for high performance sodium-ion battery anodes. Chem Commun (Camb) , 2020, 56(79): 11795 doi: 10.1039/D0CC05087C [54] Hu Z F, Lu Y L, Liu M H, et al. Crystalline red phosphorus for selective photocatalytic reduction of CO2 into CO. J Mater Chem A, 2021, 9(1): 338 doi: 10.1039/D0TA09177D [55] Hu Z F, Yuan L Y, Liu Z F, et al. An elemental phosphorus photocatalyst with a record high hydrogen evolution efficiency. Angewandte Chemie Int Ed, 2016, 55(33): 9580 doi: 10.1002/anie.201603331 [56] Wagner R S, Ellis W C. Vapor-liquid-solid mechanism of single crystal growth. Appl Phys Lett, 1964, 4(5): 89 doi: 10.1063/1.1753975 [57] Wu Y Y, Yang P D. Direct observation of vapor–liquid–solid nanowire growth. J Am Chem Soc, 2001, 123(13): 3165 doi: 10.1021/ja0059084 [58] Winchester R, Whitby M, Shaffer M. Synthesis of pure phosphorus nanostructures. Angewandte Chemie Int Ed, 2009, 48(20): 3616 doi: 10.1002/anie.200805222 [59] Liu Y, Hu Z F, Yu J C. Liquid bismuth initiated growth of phosphorus microbelts with efficient charge polarization for photocatalysis. Appl Catal B:Environ, 2019, 247: 100 doi: 10.1016/j.apcatb.2019.01.092 [60] Liu Q, Zhang X, Wang J H, et al. Crystalline red phosphorus nanoribbons: Large-scale synthesis and electrochemical nitrogen fixation. Angewandte Chemie Int Ed, 2020, 59(34): 14383 doi: 10.1002/anie.202006679 [61] Zhou J B, Liu X Y, Cai W L, et al. Wet-chemical synthesis of hollow red-phosphorus nanospheres with porous shells as anodes for high-performance lithium-ion and sodium-ion batteries. Adv Mater, 2017, 29(29): 1700214 doi: 10.1002/adma.201700214 [62] Zhang Y Y, Rui X H, Tang Y X, et al. Wet-chemical processing of phosphorus composite nanosheets for high-rate and high-capacity lithium-ion batteries. Adv Energy Mater, 2016, 6(10): 1502409 doi: 10.1002/aenm.201502409 [63] Zeng G, Hu X, Zhou B, et al. Engineering graphene with red phosphorus quantum dots for superior hybrid anodes of sodium-ion batteries. Nanoscale, 2017, 9(38): 14722 doi: 10.1039/C7NR05470J [64] Zhu J L, Liu Z G, Wang W, et al. Green, template-less synthesis of honeycomb-like porous micron-sized red phosphorus for high-performance lithium storage. ACS Nano, 2021, 15(1): 1880 doi: 10.1021/acsnano.1c00048 [65] Somayajulu M R, Gautam G K, Rao A S. Stabilisation of red phosphorus to prevent moisture absorption and suppression of phosphine release. Def Sci J, 2007, 57(6): 817 doi: 10.14429/dsj.57.1820 [66] Chang W C, Tseng K W, Tuan H Y. Solution synthesis of iodine-doped red phosphorus nanoparticles for lithium-ion battery anodes. Nano Lett, 2017, 17(2): 1240 doi: 10.1021/acs.nanolett.6b05081 [67] Liu S, Xu H, Bian X, et al. Nanoporous red phosphorus on reduced graphene oxide as superior anode for sodium-ion batteries. ACS Nano, 2018, 12(7): 7380 doi: 10.1021/acsnano.8b04075 [68] Liu S, Xu H, Bian X F, et al. Hollow nanoporous red phosphorus as an advanced anode for sodium-ion batteries. J Mater Chem A, 2018, 6(27): 12992 doi: 10.1039/C8TA03301C [69] Santhoshkumar P, Shaji N, Nanthagopal M, et al. Multichannel red phosphorus with a nanoporous architecture: A novel anode material for sodium-ion batteries. J Power Sources, 2020, 470: 228459 doi: 10.1016/j.jpowsour.2020.228459 [70] Wang F, Zi W, Zhao B X, et al. Facile solution synthesis of red phosphorus nanoparticles for lithium ion battery anodes. Nanoscale Res Lett, 2018, 13(1): 356 doi: 10.1186/s11671-018-2770-4 [71] Li Y, Jiang S, Qian Y, et al. Amine-induced phase transition from white phosphorus to red/black phosphorus for Li/K-ion storage. Chem Commun (Camb) , 2019, 55(47): 6751 doi: 10.1039/C9CC02971K [72] Liu W, Ju S, Yu X. Phosphorus-amine-based synthesis of nanoscale red phosphorus for application to sodium-ion batteries. ACS Nano, 2020, 14(1): 974 doi: 10.1021/acsnano.9b08282 [73] Liu W, Du L, Ju S, et al. Encapsulation of red phosphorus in carbon nanocages with ultrahigh content for high-capacity and long cycle life sodium-ion batteries. ACS Nano, 2021, 15(3): 5679 doi: 10.1021/acsnano.1c00924 [74] Jo M, Dragulescu-Andrasi A, Miller L Z, et al. Nucleophilic activation of red phosphorus for controlled synthesis of polyphosphides. Inorg Chem, 2020, 59(8): 5483 doi: 10.1021/acs.inorgchem.0c00108 [75] Dragulescu-Andrasi A, Miller L Z, Chen B H, et al. Facile conversion of red phosphorus into soluble polyphosphide anions by reaction with potassium ethoxide. Angewandte Chemie Int Ed, 2016, 55(12): 3904 doi: 10.1002/anie.201511186 -




 下載:
下載: