-
摘要: 鋰離子電池(LIBS)已經廣泛應用到便攜式電子產品和電動汽車上。然而,隨著鋰資源的開采使用,鋰離子電池的成本也在逐漸增加。相比之下,地殼中較高的鉀含量使得鉀離子電池(KIB)成本相對較低。進而,鉀離子電池作為一種新型低成本儲能器件受到了廣泛關注。但鉀離子的半徑較大,導致充放電過程中,離子嵌入/脫出的動力學性能較差。因此,電池電極材料的選擇面臨著新的挑戰。在對鉀離子電池電極材料進行分類和總結的基礎之上,重點介紹了石墨及各種形式的碳材料、過渡金屬氧化物、合金類等負極材料以及普魯士藍、層狀金屬氧化物、聚陰離子型化合物等正極材料的研究進展,并對鉀離子電池的發展進行了展望,以期對高性能鉀離子電池的發展提供新思路。Abstract: Development and utilization of renewable energy sources have gain great progress in recent years, which lead to increasing demands for large scale energy storage systems. Lithium-ion batteries have been widely used in portable electronic devices and electric vehicles. However, with the exploitation of the Earth’s lithium resources, the cost of lithium-ion batteries is gradually increasing. In contrast, the higher terrestrial potassium content promises inexpensive potassium-ion batteries, and the chemical properties of potassium and lithium ions are similar. Meanwhile, the low redox potential of K promises a high working voltage of potassium ion batteries. Thus, potassium-ion batteries have attracted considerable attention as a capable battery technology. However, the large radius of the potassium ion leads to unsatisfactory ion intercalation and extraction behavior during charging and discharging processes, resulting in poor cycling performance, unsatisfactory rate ability, and low capacity. The challenge remains to explore capable electrode materials for potassium-ion batteries to achieve a high energy density and power density. This review summarizes the anode and cathode materials of potassium-ion batteries in recent reports, including the research progress of graphite and other carbon materials, transition metal oxides/sulfides, alloys, and other anode materials, as well as Prussian blue, layered metal oxides, and polyanionic compound cathode materials, which will provide new ideas for developing high-performance potassium-ion batteries. We also discuss the potassium ion storage mechanism in these electrode materials. This review also demonstrates the approaches (nanotechnology, heteroatom doping, carbon coating, composite fabrication) to further improve the electrochemical performance of the cathode and anode. In addition, we point out the key factors for potassium ion batteries performance, such as the design of anode materials, exploitation of novel cathode materials, and optimization of full potassium ion cells fabrication, which would provide new thought for the development of potassium ion batteries with high performances.
-
Key words:
- potassium-ion battery /
- anode materials /
- cathode materials /
- research progress /
- high performance
-
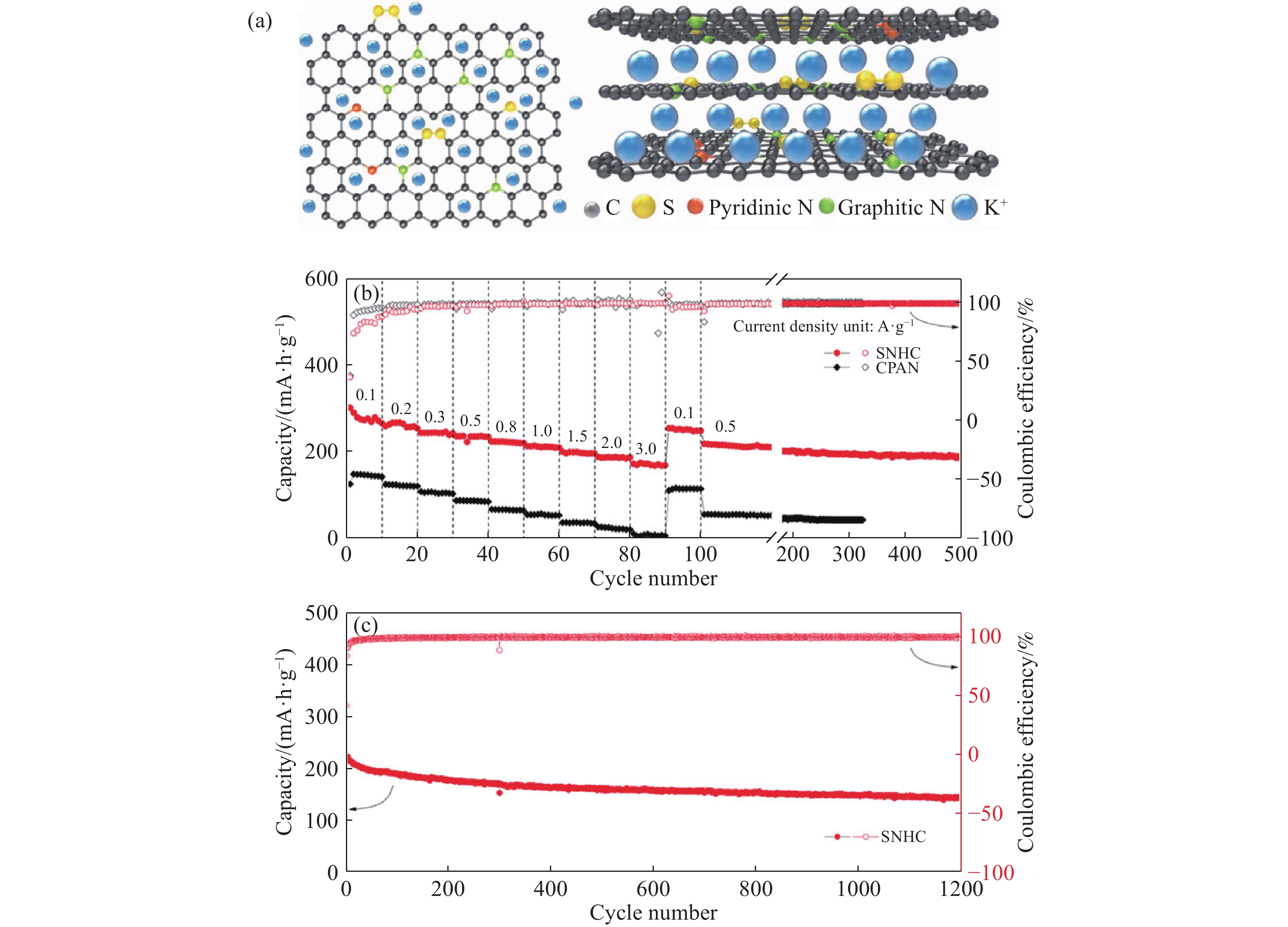
圖 3 SNHC和高溫碳化聚苯胺(CPAN)的儲鉀性能. (a)用于鉀離子電池的SNHC中大容量存儲的示意圖; (b)不同倍率下的倍率性能;(c)SNHC電極在3 A·g?1下超過1200次的循環性能[44]
Figure 3. Potassium storage properties of SNHC and CPAN: (a) schematic of bulk storage in SNHC for KIBs; (b) rate performance at different rates; (c) cycling performance of the SNHC electrodes at 3 A·g?1 over 1200 cycles [44]
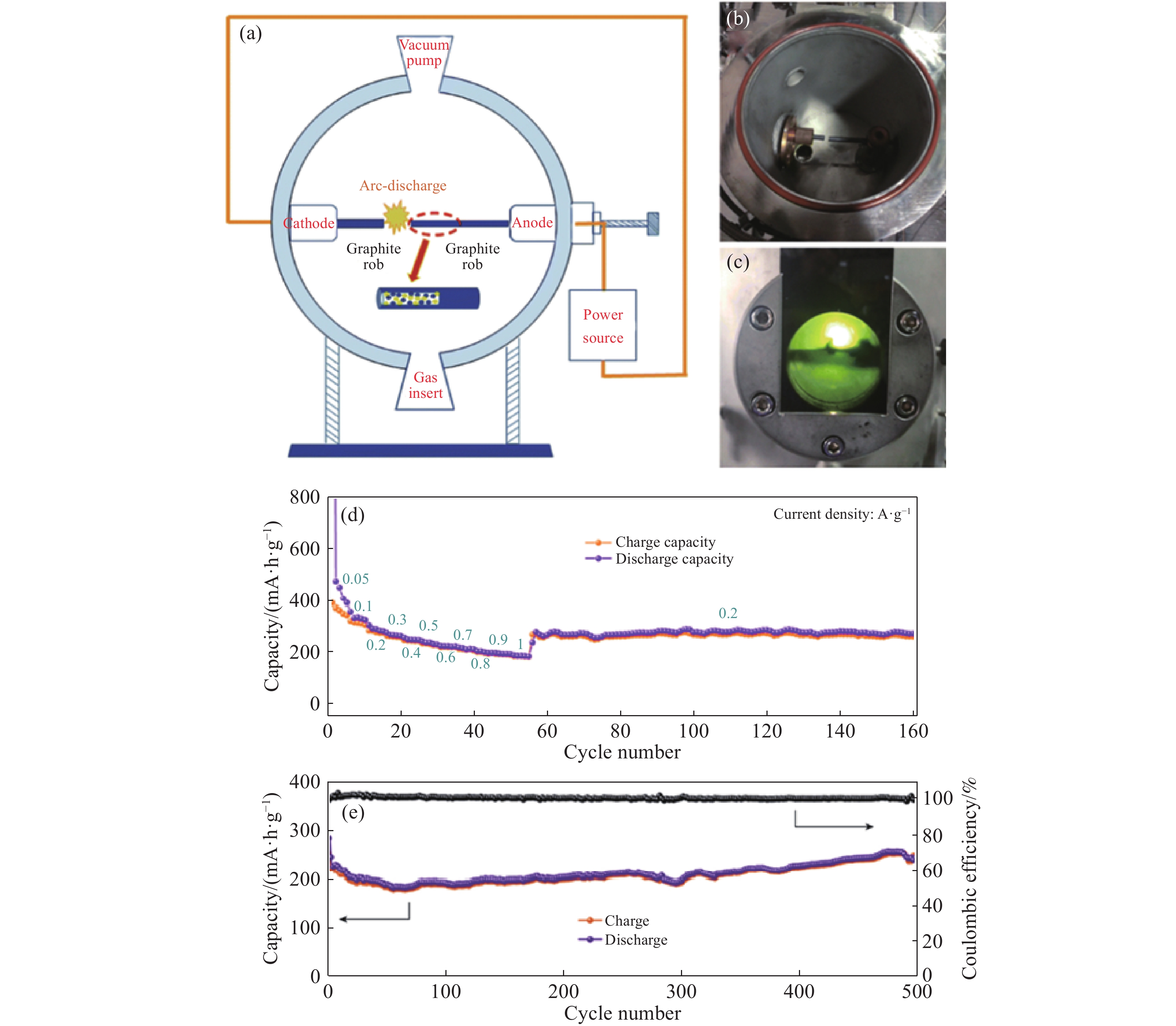
圖 4 電弧放電示意圖和NPG在鉀離子電池中的電化學性能. (a)電弧放電過程示意圖; (b)電弧放電系統圖; (c)電弧等離子區; (d)NPG電極在鉀離子電池中不同電流密度下的倍率; (e)電流密度為500 mA·g?1時NPG電極的循環和庫侖效率圖[80]
Figure 4. Schematic of discharge process and electrochemical performances of NPG at PIB: (a) schematic of the arc discharge process; (b) digital image of the arc discharge system; (c) typical photograph of the arc plasma; (d) rate capabilities of NPG electrodes in the potassium battery; (e) graph of the cycle and coulombic efficiency of NPG electrodes at a current density of 500 mA·g?1[80]
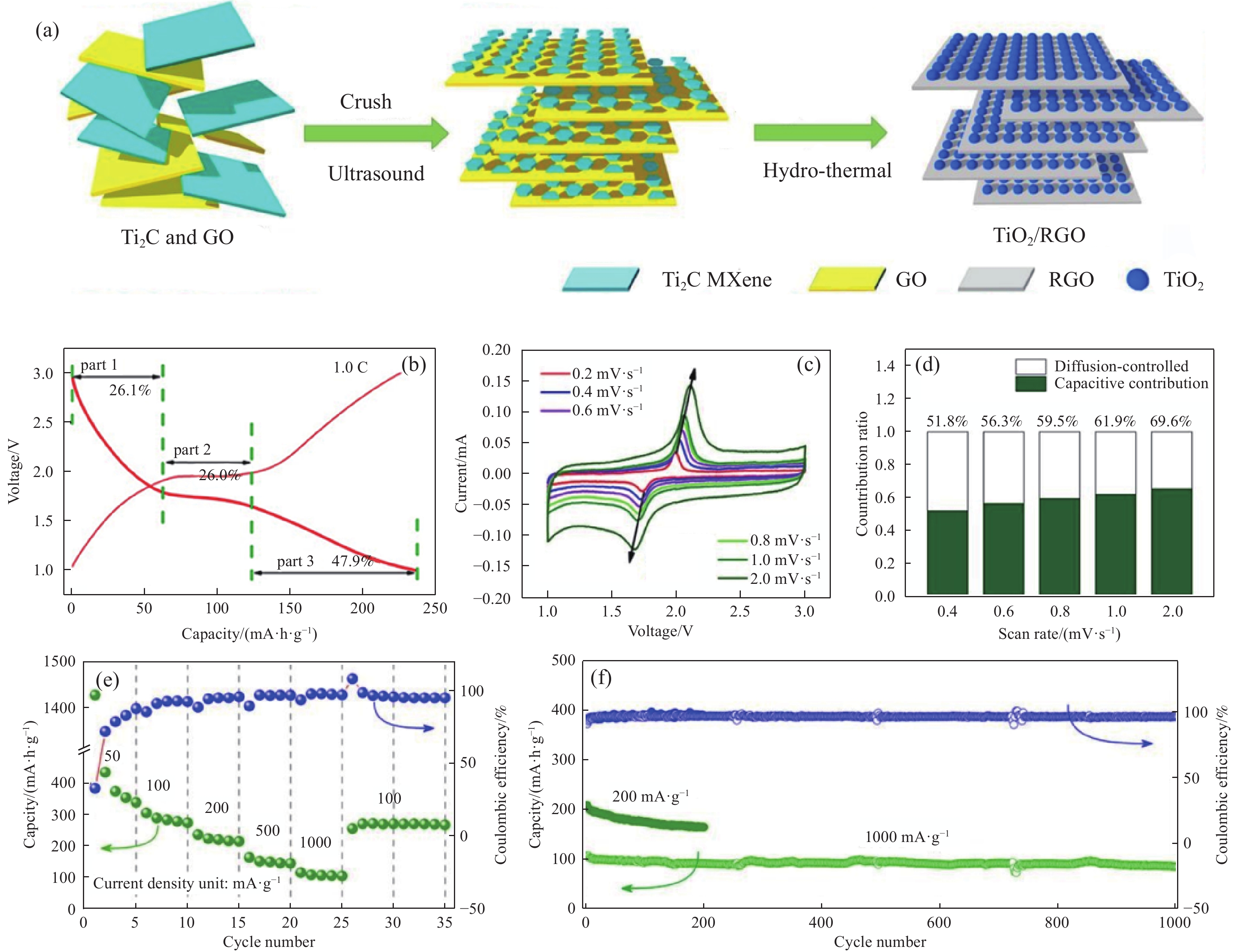
圖 5 TiO2/RGO合成示意圖和電化學性能. (a)合成示意圖; (b)在鋰離子電池中的充放電曲線; (c)循環伏安曲線; (d)贗電容貢獻; (e)鉀離子電池中的倍率性能; (f)長循環性能 [89]
Figure 5. Formation schematic and electrochemical performances of TiO2/RGO: (a) formation schematic; (b) charge-discharge curves in Li ion battery; (c) CV curves; (d) pseudocapacitance contributions; (e) rate capability in K-ion battery; (f) long cycling performance [89]
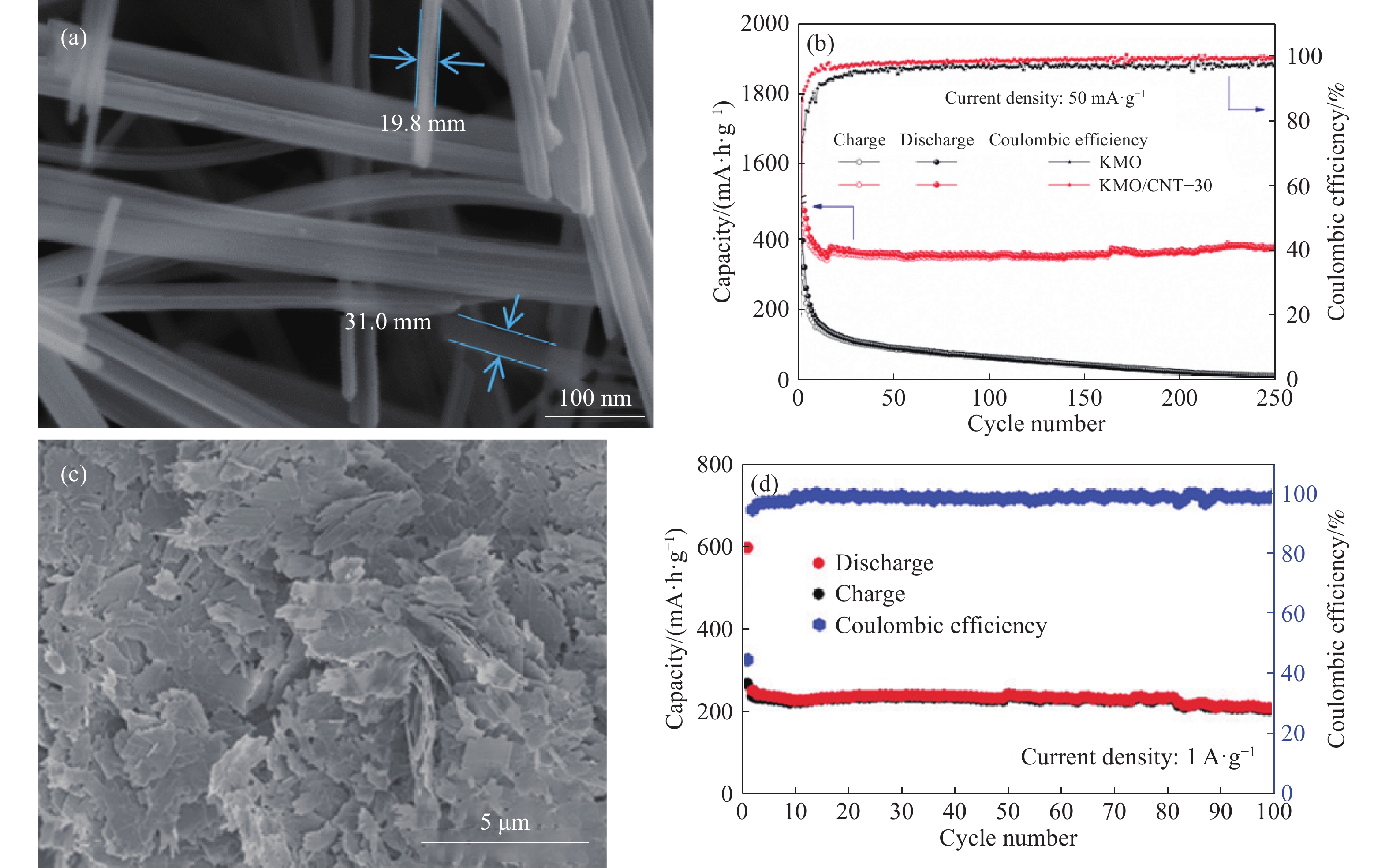
圖 6 KMO/NTS?30和CuO納米片的形貌和電化學性能. (a)KMO/NTS?30材料的SEM圖[90];(b)所制備的KMO/CNT?30電極和對照KMO電極的循環性能[90];(c)CuO納米片的SEM圖;(d)在100 mA·g?1時的循環性能 [91]
Figure 6. Morphology and electrochemical properties of KMO/NTS?30 and CuO nanosheets: (a) SEM image of KMO/NTS?30 material[90]; (b) cycling performance of the as-prepared KMO/CNT?30 electrode and the control KMO electrode[90]; (c) SEM image of CuO nanosheets; (d) cycling performance at 100 mA·g?1 [91]
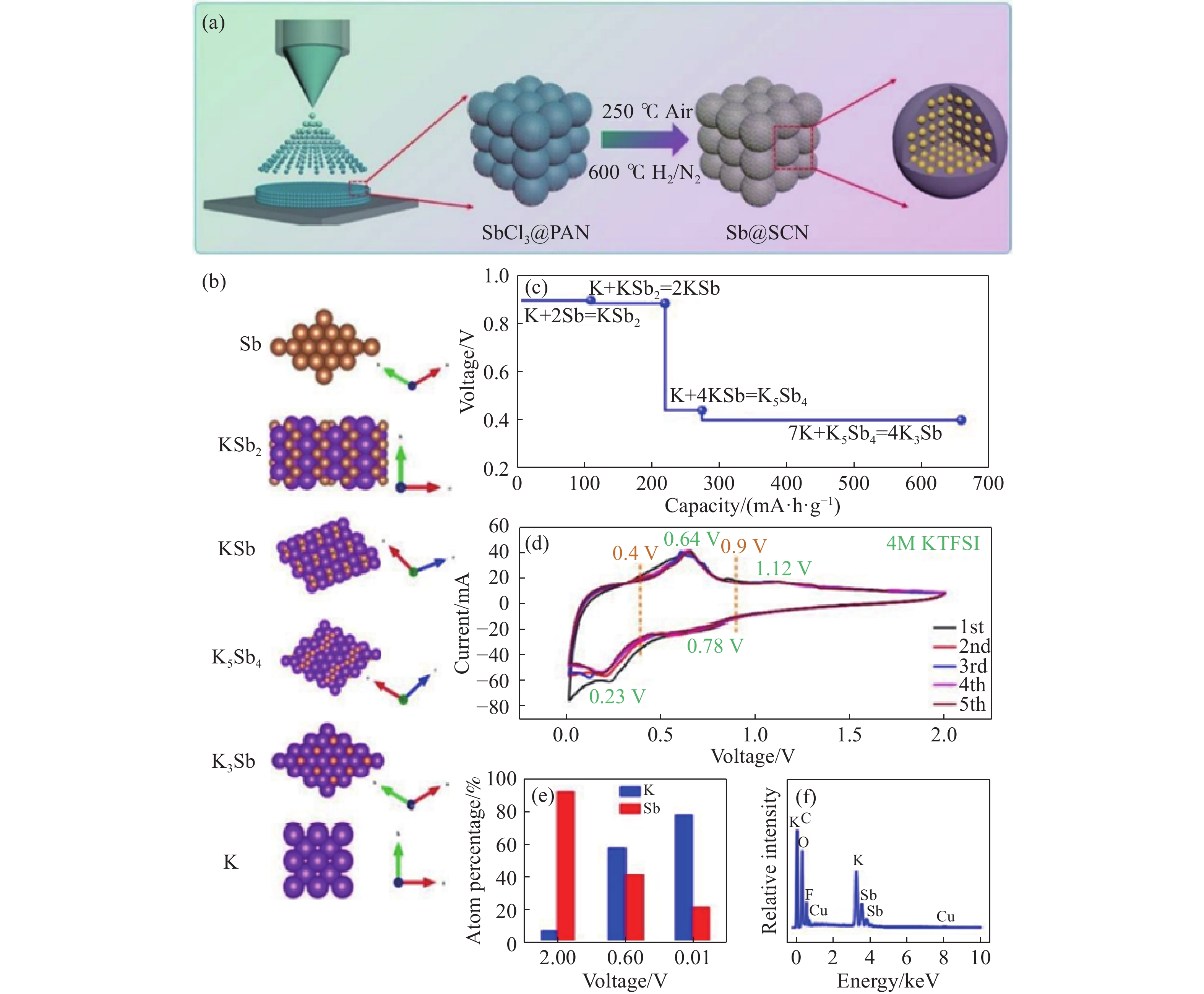
圖 8 Sb@CSN材料的合成示意圖和電化學性能與機制.(a)Sb@CSN材料的合成示意圖;(b)K的晶體結構和鉀化過程中從Sb到K3Sb的結構演變;(c)不同鉀化過程的DFT計算的平衡電壓;(d)Sb@CSN電極在0.05 mV?s?1掃描速率下的初始CV曲線;(e)從具有不同放電電壓的EDS結果獲得的K和Sb的相對原子百分比;(f)放電截止電壓為0.01V時K3Sb的EDS元素分析[111]
Figure 8. Synthetic schematic, electrochemical properties and mechanisms of Sb@CSN material: (a) synthetic schematic; (b) crystal structure of K and the structure evolution from Sb to K3Sb during the potassiation process; (c) equilibrium voltages calculated by DFT of different potassiation processes; (d) initial CV curves of the Sb@CSN electrode at a scan rate of 0.05 mV?s?1; (e) relative atomic percent of K and Sb obtained from EDS results with different discharge voltages; (f) EDS element analysis for K3Sb with a discharge cut-off voltage of 0.01 V [111]
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Lu J, Wu T P, Amine K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat Energy, 2017, 2: 17011 doi: 10.1038/nenergy.2017.11 [2] Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices. Science, 2011, 334(6058): 928 doi: 10.1126/science.1212741 [3] Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature, 2001, 414(6861): 359 doi: 10.1038/35104644 [4] Armand M, Tarascon J M. Building better batteries. Nature, 2008, 451(7179): 652 doi: 10.1038/451652a [5] Han M H, Gonzalo E, Singh G, et al. A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ Sci, 2015, 8(1): 81 doi: 10.1039/C4EE03192J [6] Xie D H, Zhang M, Wu Y, et al. A flexible dual-ion battery based on sodium-ion quasi-solid-state electrolyte with long cycling life. Adv Funct Mater, 2020, 30(5): 1906770 doi: 10.1002/adfm.201906770 [7] Zhang W C, Liu Y J, Guo Z P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci Adv, 2019, 5(5): eaav7412 doi: 10.1126/sciadv.aav7412 [8] Xu Y, Bahmani F, Zhou M, et al. Enhancing potassium-ion battery performance by defect and interlayer engineering. Nanoscale Horiz, 2019, 4(1): 202 doi: 10.1039/C8NH00305J [9] Guo J Z, Gu Z Y, Zhao X X, et al. Flexible Na/K-ion full batteries from the renewable cotton cloth-derived stable, low-cost, and binder-free anode and cathode. Adv Energy Mater, 2019, 9(38): 1902056 doi: 10.1002/aenm.201902056 [10] Dhir S, Wheeler S, Capone I, et al. Outlook on K-ion batteries. Chem, 2020, 6(10): 2442 doi: 10.1016/j.chempr.2020.08.012 [11] Wang J, Fan L, Liu Z M, et al. In situ alloying strategy for exceptional potassium ion batteries. ACS Nano, 2019, 13(3): 3703 doi: 10.1021/acsnano.9b00634 [12] Komaba S, Hasegawa T, Dahbi M, et al. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem Commun, 2015, 60: 172 doi: 10.1016/j.elecom.2015.09.002 [13] Jian Z L, Luo W, Ji X L. Carbon electrodes for K-ion batteries. J Am Chem Soc, 2015, 137(36): 11566 doi: 10.1021/jacs.5b06809 [14] Okoshi M, Yamada Y, Komaba S, et al. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: A comparison with lithium, sodium, and magnesium ions. J Electrochem Soc, 2016, 164(2): A54 [15] Lei K X, Li F J, Mu C N, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ Sci, 2017, 10(2): 552 doi: 10.1039/C6EE03185D [16] Chang X Q, Zhou X L, Ou X W, et al. Ultrahigh nitrogen doping of carbon nanosheets for high capacity and long cycling potassium ion storage. Adv Energy Mater, 2019, 9(47): 1902672 doi: 10.1002/aenm.201902672 [17] Hwang J Y, Myung S T, Sun Y K. Recent progress in rechargeable potassium batteries. Adv Functi Mater, 2018, 28(43): 1802938 doi: 10.1002/adfm.201802938 [18] Yi Z, Lin N, Zhang W Q, et al. Preparation of Sb nanoparticles in molten salt and their potassium storage performance and mechanism. Nanoscale, 2018, 10(27): 13236 doi: 10.1039/C8NR03829E [19] Kubota K, Dahbi M, Hosaka T, et al. Towards k-ion and na-ion batteries as “Beyond Li-ion”. Chem Rec, 2018, 18(4): 459 doi: 10.1002/tcr.201700057 [20] Dresselhaus M S, Dresselhaus G. Intercalation compounds of graphite. Adv Phys, 1981, 30(2): 139 doi: 10.1080/00018738100101367 [21] Luo W, Wan J Y, Ozdemir B, et al. Potassium ion batteries with graphitic materials. Nano Lett, 2015, 15(11): 7671 doi: 10.1021/acs.nanolett.5b03667 [22] Liu J L, Yin T T, Tian B B, et al. Unraveling the potassium storage mechanism in graphite foam. Adv Energy Mater, 2019, 9(22): 1900579 doi: 10.1002/aenm.201900579 [23] Xing Z Y, Qi Y T, Jian Z L, et al. Polynanocrystalline graphite: A new carbon anode with superior cycling performance for K-ion batteries. ACS Appl Mater Interfaces, 2017, 9(5): 4343 doi: 10.1021/acsami.6b06767 [24] An Y L, Fei H, Zeng G, et al. Commercial expanded graphite as a low–cost, long-cycling life anode for potassium–ion batteries with conventional carbonate electrolyte. J Power Sources, 2018, 378: 66 doi: 10.1016/j.jpowsour.2017.12.033 [25] Carboni M, Naylor A J, Valvo M, et al. Unlocking high capacities of graphite anodes for potassium-ion batteries. RSC Adv, 2019, 9(36): 21070 doi: 10.1039/C9RA01931F [26] Rahman M M, Hou C, Mateti S, et al. Documenting capacity and cyclic stability enhancements in synthetic graphite potassium-ion battery anode material modified by low-energy liquid phase ball milling. J Power Sources, 2020, 476: 228733 doi: 10.1016/j.jpowsour.2020.228733 [27] Jiang J M, Nie G D, Nie P, et al. Nanohollow carbon for rechargeable batteries: Ongoing progresses and challenges. Nanomicro Lett, 2020, 12(1): 183 [28] Feng Y H, Chen S H, Shen D Y, et al. Cross-linked hollow graphitic carbon as low-cost and high-performance anode for potassium ion batteries. Energy &Environ Mater, 2021, 4(3): 451 [29] Cao B, Zhang Q, Liu H, et al. Graphitic carbon nanocage as a stable and high power anode for potassium-ion batteries. Adv Energy Mater, 2018, 8(25): 1801149 doi: 10.1002/aenm.201801149 [30] Zhang W L, Ming J, Zhao W L, et al. Graphitic nanocarbon with engineered defects for high-performance potassium-ion battery anodes. Adv Funct Mater, 2019, 29(35): 1903641 doi: 10.1002/adfm.201903641 [31] Kim J, Choi M S, Shin K H, et al. Rational design of carbon nanomaterials for electrochemical sodium storage and capture. Adv Mater, 2019, 31(34): e1803444 doi: 10.1002/adma.201803444 [32] Xiao B W, Rojo T, Li X L. Hard carbon as sodium-ion battery anodes: progress and challenges. ChemSusChem, 2019, 12(1): 133 doi: 10.1002/cssc.201801879 [33] Dou X W, Hasa I, Saurel D, et al. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater Today, 2019, 23: 87 doi: 10.1016/j.mattod.2018.12.040 [34] Lee H H, Wan C C, Wang Y Y. Identity and thermodynamics of lithium intercalated in graphite. J Power Sources, 2003, 114(2): 285 doi: 10.1016/S0378-7753(02)00606-7 [35] Dahn J R, Zheng T, Liu Y H, et al. Mechanisms for lithium insertion in carbonaceous materials. Science, 1995, 270(5236): 590 doi: 10.1126/science.270.5236.590 [36] Luo W, Jian Z L, Xing Z Y, et al. Electrochemically expandable soft carbon as anodes for Na-ion batteries. ACS Cent Sci, 2015, 1(9): 516 doi: 10.1021/acscentsci.5b00329 [37] Wang X P, Han K, Qin D D, et al. Polycrystalline soft carbon semi-hollow microrods as anode for advanced K-ion full batteries. Nanoscale, 2017, 9(46): 18216 doi: 10.1039/C7NR06645G [38] Bin D S, Li Y M, Sun Y G, et al. Structural engineering of multishelled hollow carbon nanostructures for high-performance Na-ion battery anode. Adv Energy Mater, 2018, 8(26): 1800855 doi: 10.1002/aenm.201800855 [39] Jian Z L, Xing Z Y, Bommier C, et al. Hard carbon microspheres: Potassium-ion anode versus sodium-ion anode. Adv Energy Mater, 2016, 6(3): 1501874 doi: 10.1002/aenm.201501874 [40] Chen C J, Wang Z, Zhang B, et al. Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Storage Mater, 2017, 8: 161 doi: 10.1016/j.ensm.2017.05.010 [41] Jian Z L, Hwang S, Li Z F, et al. Hard-soft composite carbon as a long-cycling and high-rate anode for potassium-ion batteries. Adv Funct Mater, 2017, 27(26): 1700324 doi: 10.1002/adfm.201700324 [42] Tai Z X, Zhang Q, Liu Y, et al. Activated carbon from the graphite with increased rate capability for the potassium ion battery. Carbon, 2017, 123: 54 doi: 10.1016/j.carbon.2017.07.041 [43] Zhang Q F, Cheng X L, Wang C X, et al. Sulfur-assisted large-scale synthesis of graphene microspheres for superior potassium-ion batteries. Energy Environ Sci, 2021, 14(2): 965 doi: 10.1039/D0EE03203D [44] Liu Y, Dai H D, Wu L, et al. A large scalable and low-cost sulfur/nitrogen dual-doped hard carbon as the negative electrode material for high-performance potassium-ion batteries. Adv Energy Mater, 2019, 9(34): 1901379 doi: 10.1002/aenm.201901379 [45] Lu C, Sun Z T, Yu L H, et al. Enhanced kinetics harvested in heteroatom dual-doped graphitic hollow architectures toward high rate printable potassium-ion batteries. Adv Energy Mater, 2020, 10(28): 2001161 doi: 10.1002/aenm.202001161 [46] Ma M Z, Zhang S P, Yao Y, et al. Heterostructures of 2D molybdenum dichalcogenide on 2D nitrogen-doped carbon: Superior potassium-ion storage and insight into potassium storage mechanism. Adv Mater, 2020, 32(22): e2000958 doi: 10.1002/adma.202000958 [47] Ferrero G A, Preuss K, Marinovic A, et al. Fe-N-Doped carbon capsules with outstanding electrochemical performance and stability for the oxygen reduction reaction in both acid and alkaline conditions. ACS Nano, 2016, 10(6): 5922 doi: 10.1021/acsnano.6b01247 [48] Wu J X, Pan Z Y, Zhang Y, et al. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J Mater Chem A, 2018, 6(27): 12932 doi: 10.1039/C8TA03968B [49] Hong Z S, Maleki H, Ludwig T, et al. New insights into carbon-based and MXene anodes for Na and K-ion storage: A review. J Energy Chem, 2021, 62: 660 doi: 10.1016/j.jechem.2021.04.031 [50] Yang W X, Zhou J H, Wang S, et al. Freestanding film made by necklace-like N-doped hollow carbon with hierarchical pores for high-performance potassium-ion storage. Energy Environ Sci, 2019, 12(5): 1605 doi: 10.1039/C9EE00536F [51] Zhang W L, Cao Z, Wang W X, et al. A site-selective doping strategy of carbon anodes with remarkable K-ion storage capacity. Angew Chem Int Ed, 2020, 59(11): 4448 doi: 10.1002/anie.201913368 [52] Zhang W L, Yin J, Sun M L, et al. Direct pyrolysis of supermolecules: An ultrahigh edge-nitrogen doping strategy of carbon anodes for potassium-ion batteries. Adv Mater, 2020, 32(25): e2000732 doi: 10.1002/adma.202000732 [53] Xu Y, Zhang C L, Zhou M, et al. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat Commun, 2018, 9(1): 1720 doi: 10.1038/s41467-018-04190-z [54] Huang H J, Xu R, Feng Y Z, et al. Sodium/Potassium-ion batteries: Boosting the rate capability and cycle life by combining morphology, defect and structure engineering. Adv Mater, 2020, 32(8): e1904320 doi: 10.1002/adma.201904320 [55] Yan J, Li H M, Wang K L, et al. Ultrahigh phosphorus doping of carbon for high-rate sodium ion batteries anode. Adv Energy Mater, 2021, 11(21): 2003911 doi: 10.1002/aenm.202003911 [56] Qian J F, Wu X Y, Cao Y L, et al. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew Chem Int Ed, 2013, 52(17): 4633 doi: 10.1002/anie.201209689 [57] Kim Y, Park Y, Choi A, et al. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv Mater, 2013, 25(22): 3045 doi: 10.1002/adma.201204877 [58] Sun Y M, Wang L, Li Y, et al. Design of red phosphorus nanostructured electrode for fast-charging lithium-ion batteries with high energy density. Joule, 2019, 3(4): 1080 doi: 10.1016/j.joule.2019.01.017 [59] Liu Y H, Liu Q Z, Jian C, et al. Red-phosphorus-impregnated carbon nanofibers for sodium-ion batteries and liquefaction of red phosphorus. Nat Commun, 2020, 11: 2520 doi: 10.1038/s41467-020-16077-z [60] Xiong P X, Bai P X, Tu S B, et al. Red phosphorus nanoparticle@3D interconnected carbon nanosheet framework composite for potassium-ion battery anodes. Small, 2018: e1802140 [61] Liu D, Huang X K, Qu D Y, et al. Confined phosphorus in carbon nanotube-backboned mesoporous carbon as superior anode material for sodium/potassium-ion batteries. Nano Energy, 2018, 52: 1 doi: 10.1016/j.nanoen.2018.07.023 [62] Xiao W, Li X F, Cao B, et al. Constructing high-rate and long-life phosphorus/carbon anodes for potassium-ion batteries through rational nanoconfinement. Nano Energy, 2021, 83: 105772 doi: 10.1016/j.nanoen.2021.105772 [63] Li J L, Qin W, Xie J, et al. Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage. Nano Energy, 2018, 53: 415 doi: 10.1016/j.nanoen.2018.08.075 [64] Ding J, Zhang H, Zhou H, et al. Sulfur-grafted hollow carbon spheres for potassium-ion battery anodes. Adv Mater, 2019, 31(30): e1900429 [65] Tian S, Guan D, Lu J, et al. Synthesis of the electrochemically stable sulfur-doped bamboo charcoal as the anode material of potassium-ion batteries. J Power Sources, 2020, 448: 227572 doi: 10.1016/j.jpowsour.2019.227572 [66] Xie X, Qi S, Wu D, et al. Porous surfur-doped hard carbon for excellent potassium storage. Chin Chem Lett, 2020, 31(1): 223 doi: 10.1016/j.cclet.2019.10.008 [67] Long W Y, Fang B Z, Ignaszak A, et al. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem Soc Rev, 2017, 46(23): 7176 doi: 10.1039/C6CS00639F [68] Chen W M, Wan M, Liu Q, et al. Heteroatom-doped carbon materials: Synthesis, mechanism, and application for sodium-ion batteries. Small Methods, 2019, 3(4): 1800323 doi: 10.1002/smtd.201800323 [69] Xia G L, Wang C L, Jiang P, et al. Nitrogen/oxygen co-doped mesoporous carbon octahedrons for high-performance potassium-ion batteries. J Mater Chem A, 2019, 7(19): 12317 doi: 10.1039/C8TA12504J [70] Cui R C, Xu B, Dong H J, et al. N/O dual-doped environment-friendly hard carbon as advanced anode for potassium-ion batteries. Adv Sci, 2020, 7(5): 1902547 doi: 10.1002/advs.201902547 [71] Zhu Y Y, Wang M, Zhang Y, et al. Nitrogen/oxygen dual-doped hierarchically porous carbon/graphene composite as high-performance anode for potassium storage. Electrochimica Acta, 2021, 377: 138093 doi: 10.1016/j.electacta.2021.138093 [72] Wang L F, Li S, Li J, et al. Nitrogen/sulphur co-doped porous carbon derived from wasted wet wipes as promising anode material for high performance capacitive potassium-ion storage. Mater Today Energy, 2019, 13: 195 doi: 10.1016/j.mtener.2019.05.010 [73] Li Y P, Zhong W, Yang C, et al. N/S codoped carbon microboxes with expanded interlayer distance toward excellent potassium storage. Chem Eng J, 2019, 358: 1147 doi: 10.1016/j.cej.2018.10.135 [74] Ma H L, Qi X J, Peng D Q, et al. Novel fabrication of N/S Co-doped hierarchically porous carbon for potassium-ion batteries. Chemistry Select, 2019, 4(39): 11488 [75] He H N, Huang D, Tang Y, et al. Tuning nitrogen species in three-dimensional porous carbon via phosphorus doping for ultra-fast potassium storage. Nano Energy, 2019, 57: 728 doi: 10.1016/j.nanoen.2019.01.009 [76] Gan Q M, Xie J W, Zhu Y H, et al. Sub-20 nm carbon nanoparticles with expanded interlayer spacing for high-performance potassium storage. ACS Appl Mater Interfaces, 2019, 11(1): 930 doi: 10.1021/acsami.8b18553 [77] Zhang Z L, Jia B R, Liu L, et al. Hollow multihole carbon bowls: A stress-release structure design for high-stability and high-volumetric-capacity potassium-ion batteries. ACS Nano, 2019, 13(10): 11363 doi: 10.1021/acsnano.9b04728 [78] Ruan J F, Zhao Y, Luo S, et al. Fast and stable potassium-ion storage achieved by in situ molecular self-assembling N/O dual-doped carbon network. Energy Storage Mater, 2019, 23: 46 doi: 10.1016/j.ensm.2019.05.037 [79] Lu X L, Pan X N, Fang Z, et al. High-performance potassium-ion batteries with robust stability based on N/S-codoped hollow carbon nanocubes. ACS Appl Mater Interfaces, 2021, 13(35): 41619 doi: 10.1021/acsami.1c10655 [80] Luan Y T, Hu R, Fang Y Z, et al. Nitrogen and phosphorus dual-doped multilayer graphene as universal anode for full carbon-based lithium and potassium ion capacitors. Nanomicro Lett, 2019, 11(1): 30 [81] Kishore B, Venkatesh G, Munichandraiah N. K2Ti4O9: A promising anode material for potassium ion batteries. J Electrochem Soc, 2016, 163(13): A2551 doi: 10.1149/2.0421613jes [82] Xu S M, Liu X, Zhang Q, et al. Boosting potassium storage capacity based on stress-induced size-dependent solid-solution behavior. Adv Energy Mater, 2018, 8(32): 1802175 doi: 10.1002/aenm.201802175 [83] Han J, Xu M W, Niu Y B, et al. Exploration of K2Ti8O17 as an anode material for potassium-ion batteries. Chem Commun, 2016, 52(75): 11274 doi: 10.1039/C6CC05102B [84] Li Y P, Yang C H, Zheng F H, et al. Design of TiO2eC hierarchical tubular heterostructures for high performance potassium ion batteries. Nano Energy, 2019, 59: 582 doi: 10.1016/j.nanoen.2019.03.002 [85] Han J, Niu Y B, Bao S J, et al. Nanocubic KTi2(PO4)3 electrodes for potassium-ion batteries. Chem Commun, 2016, 52(78): 11661 doi: 10.1039/C6CC06177J [86] Er D Q, Li J W, Naguib M, et al. Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl Mater Int, 2014, 6(14): 11173 doi: 10.1021/am501144q [87] Lian P C, Dong Y, Wu Z S, et al. Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy, 2017, 40: 1 doi: 10.1016/j.nanoen.2017.08.002 [88] Dong Y F, Wu Z S, Zheng S H, et al. Ti3C2 MXene-derived sodium/potassium titanate nanoribbons for high-performance sodium/potassium ion batteries with enhanced capacities. ACS Nano, 2017, 11(5): 4792 doi: 10.1021/acsnano.7b01165 [89] Fang Y Z, Hu R, Liu B Y, et al. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries. J Mater Chem A, 2019, 7(10): 5363 doi: 10.1039/C8TA12069B [90] Chong S K, Wu Y, Liu C, et al. Cryptomelane-type MnO2/carbon nanotube hybrids as bifunctional electrode material for high capacity potassium-ion full batteries. Nano Energy, 2018, 54: 106 doi: 10.1016/j.nanoen.2018.09.072 [91] Cao K Z, Liu H Q, Li W Y, et al. CuO nanoplates for high-performance potassium-ion batteries. Small, 2019, 15(36): e1901775 doi: 10.1002/smll.201901775 [92] Chen F, Wang S, He X D, et al. Hollow sphere structured V2O3@C as an anode material for high capacity potassium-ion batteries. J Mater Chem A, 2020, 8(26): 13261 doi: 10.1039/D0TA01057J [93] Niu X G, Zhang Y, Tan L, et al. Amorphous FeVO4 as a promising anode material for potassium-ion batteries. Energy Storage Mater, 2019, 22: 160 doi: 10.1016/j.ensm.2019.01.011 [94] Sun D, Ye D L, Liu P, et al. MoS2/graphene nanosheets from commercial bulky MoS2 and graphite as anode materials for high rate sodium-ion batteries. Adv Energy Mater, 2018, 8(10): 1702383 doi: 10.1002/aenm.201702383 [95] Azhagurajan M, Kajita T, Itoh T, et al. In situ visualization of lithiumlon intercalation into MoS2 single crystals using differential optical microscopy with atomic layer resolution. J Am Chem Soc, 2016, 138(10): 3355 doi: 10.1021/jacs.5b11849 [96] Wang L L, Zhang Q, Zhu J, et al. Nature of extra capacity in MoS2 electrodes: Molybdenum atoms accommodate with lithium. Energy Storage Mater, 2019, 16: 37 doi: 10.1016/j.ensm.2018.04.025 [97] Ren X D, Zhao Q, McCulloch W D, et al. MoS2 as a long-life host material for potassium ion intercalation. Nano Res, 2017, 10(4): 1313 doi: 10.1007/s12274-016-1419-9 [98] Xie K Y, Yuan K, Li X, et al. Superior potassium ion storage via vertical MoS2 “Nano-Rose” with expanded interlayers on grapheme. Small, 2017, 13(42): 1701471 doi: 10.1002/smll.201701471 [99] Bai J, Xi B J, Mao H Z, et al. One-step construction of N, P-codoped porous carbon sheets/CoP hybrids with enhanced lithium and potassium storage. Adv Mater, 2018, 30(35): e1802310 doi: 10.1002/adma.201802310 [100] Lao M M, Zhang Y, Luo W B, et al. Alloy-based anode materials toward advanced sodium-Ion batteries. Adv Mater, 2017, 29(48): 1700622 doi: 10.1002/adma.201700622 [101] Sultana I, Rahman M M, Chen Y, et al. Potassium-ion battery anode materials operating through the alloying-dealloying reaction mechanism. Adv Funct Mater, 2018, 28(5): 1703857 doi: 10.1002/adfm.201703857 [102] Sultana I, Ramireddy T, Rahman M M, et al. Tin-based composite anodes for potassium-ion batteries. Chem Commun, 2016, 52(59): 9279 doi: 10.1039/C6CC03649J [103] Wang Q N, Zhao X X, Ni C L, et al. Reaction and capacity-fading mechanisms of tin nanoparticles in potassium-ion batteries. J Phys Chem C, 2017, 121(23): 12652 doi: 10.1021/acs.jpcc.7b03837 [104] Wang H, Xing Z, Hu Z, et al. Sn-based submicron-particles encapsulated in porous reduced graphene oxide network: Advanced anodes for high-rate and long life potassium-ion batteries. Appl Mater Today, 2019, 15: 58 doi: 10.1016/j.apmt.2018.12.020 [105] Huang K S, Xing Z, Wang L C, et al. Direct synthesis of 3D hierarchically porous carbon/Sn composites via in situ generated NaCl crystals as templates for potassium-ion batteries anode. J Mater Chem A, 2018, 6(2): 434 doi: 10.1039/C7TA08171E [106] Luo S C, Wang T Y, Lu H Y, et al. Ultrasmall SnO2 nanocrystals embedded in porous carbon as potassium ion battery anodes with long-term cycling performance. New J Chem, 2020, 44(27): 11678 doi: 10.1039/D0NJ00323A [107] Zhang W C, Mao J F, Li S A, et al. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J Am Chem Soc, 2017, 139(9): 3316 doi: 10.1021/jacs.6b12185 [108] Li D P, Sun Q, Zhang Y M, et al. Surface-confined SnS2@C@rGO as high-performance anode materials for sodium- and potassium-ion batteries. ChemSusChem, 2019, 12(12): 2689 doi: 10.1002/cssc.201900719 [109] McCulloch W D, Ren X D, Yu M Z, et al. Potassium-ion oxygen battery based on a high capacity antimony anode. ACS Appl Mater Interfaces, 2015, 7(47): 26158 doi: 10.1021/acsami.5b08037 [110] Han C H, Han K, Wang X P, et al. Three-dimensional carbon network confined antimony nanoparticle anodes for high-capacity K-ion batteries. Nanoscale, 2018, 10(15): 6820 doi: 10.1039/C8NR00237A [111] Zheng J, Yang Y, Fan X L, et al. Extremely stable antimony–carbon composite anodes for potassium-ion batteries. Energy Environ Sci, 2019, 12(2): 615 doi: 10.1039/C8EE02836B [112] Lei K X, Wang C C, Liu L J, et al. A porous network of bismuth used as the anode material for high-energy-density potassium-ion batteries. Angew Chem Int Ed, 2018, 57(17): 4687 doi: 10.1002/anie.201801389 [113] Zhang Q, Mao J F, Pang W K, et al. Boosting the potassium storage performance of alloy-based anode materials via electrolyte salt chemistry. Adv Energy Mater, 2018, 8(15): 1703288 doi: 10.1002/aenm.201703288 [114] Shen C, Cheng T L, Liu C Y, et al. Bismuthene from sonoelectrochemistry as a superior anode for potassium-ion batteries. J Mater Chem A, 2020, 8(1): 453 doi: 10.1039/C9TA11000C [115] Zhao Y X, Ren X C, Xing Z J, et al. In situ formation of hierarchical Bismuth nanodots/graphene nanoarchitectures for ultrahigh-rate and durable potassium-ion storage. Small, 2020, 16(2): e1905789 doi: 10.1002/smll.201905789 [116] Li H, Zhao C X, Yin Y M, et al. N-Doped carbon coated bismuth nanorods with a hollow structure as an anode for superior-performance potassium-ion batteries. Nanoscale, 2020, 12(7): 4309 doi: 10.1039/C9NR09867D [117] Eftekhari A. Potassium secondary cell based on Prussian blue cathode. J Power Sources, 2004, 126(1-2): 221 doi: 10.1016/j.jpowsour.2003.08.007 [118] Targholi E, Mousavi-Khoshdel S M, Rahmanifara M, et al. Cu- and Fe-hexacyanoferrate as cathode materials for Potassium ion battery: A First-principles study. Chem Phy Lett, 2017, 687: 244 doi: 10.1016/j.cplett.2017.09.029 [119] Lu Y H, Wang L, Cheng J, Goodenough J B. Prussian blue: A new framework of electrode materials for sodium batteries. Chemical Commun, 2012, 48(52): 6544 doi: 10.1039/c2cc31777j [120] Wu X Y, Leonard D P, Ji X L. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities. Chem Mater, 2017, 29(12): 5031 doi: 10.1021/acs.chemmater.7b01764 [121] Xue L G, Gao H C, Zhou W D, et al. Liquid K-Na alloy anode enables dendrite-free potassium batteries. Adv Mater, 2016, 28(43): 9608 doi: 10.1002/adma.201602633 [122] Xue L G, Li Y T, Gao H C, et al. Low-cost high-energy potassium cathode. J Am Chem Soc, 2017, 139(6): 2164 doi: 10.1021/jacs.6b12598 [123] Shaju K M, Bruce P G. Macroporous Li(Ni1/3Co1/3Mn1/3)O2: A high-power and high-energy cathode for rechargeable lithium batteries. Adv Mater, 2006, 18(17): 2330 doi: 10.1002/adma.200600958 [124] Naveen N, Park W B, Han S C, et al. Reversible K+-insertion/deinsertion and concomitant Na+-redistribution in P’3-Na0.52CrO2 for high-performance potassium-ion battery cathodes. Chem Mater, 2018, 30(6): 2049 doi: 10.1021/acs.chemmater.7b05329 [125] Bo S H, Li X, Toumar A J, et al. Layered-to-rock-salt transformation in desodiated NaxCrO2 (x 0.4). Chem Mater, 2016, 28(5): 1419 doi: 10.1021/acs.chemmater.5b04626 [126] Zhao S Q, Yan K, Munroe P, et al. Construction of hierarchical K1.39Mn3O6 spheres via AlF3 coating for high-performance potassium-ion batteries. Adv Energy Mater, 2019, 9(10): 1803757 doi: 10.1002/aenm.201803757 [127] Delmas C, Braconnier J J, Fouassier C, et al. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion, 1981, 3–4: 165 [128] Kim H, Seo D H, Urban A, et al. Stoichiometric layered potassium transition metal oxide for rechargeable potassium batteries. Chem Mater, 2018, 30(18): 6532 doi: 10.1021/acs.chemmater.8b03228 [129] Zhang H Y, Xi K Y, Jiang K Z, et al. Enhanced K-ion kinetics in a layered cathode for potassium ion batteries. Chem Commun, 2019, 55(55): 7910 doi: 10.1039/C9CC03156A [130] Saravanan K, Mason C W, Rudola A, et al. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv Energy Mater, 2013, 3(4): 444 doi: 10.1002/aenm.201200803 [131] Recham N, Chotard J N, Dupont L, et al. A 3.6 V lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries. Nat Mater, 2010, 9(1): 68 [132] Wang Y G, Wang Y R, Hosono E, et al. The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed, 2008, 47(39): 7461 doi: 10.1002/anie.200802539 [133] Kim H, Kim J C, Bo S H, et al. K-ion batteries based on a P2-type K0.6CoO2 cathode. Adv Energy Mater, 2017, 7(17): 1700098 doi: 10.1002/aenm.201700098 [134] Kim H, Seo D H, Bianchini M, et al. A new strategy for high-voltage cathodes for K-ion batteries: Stoichiometric KVPO4F. Adv Energy Mater, 2018, 8(26): 1801591 doi: 10.1002/aenm.201801591 [135] Masquelier C, Croguennec L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem Rev, 2013, 113(8): 6552 doi: 10.1021/cr3001862 [136] Hatakeyama-Sato K, Akahane T, Go C, et al. Ultrafast charge/discharge by a 99.9% conventional lithium iron phosphate electrode containing 0. 1% redox-active fluoflavin polymer. ACS Energy Lett, 2020, 5(5): 1712 [137] Li J K, Ma Z F. Past and present of LiFePO4: From fundamental research to industrial applications. Chem, 2019, 5(1): 3 doi: 10.1016/j.chempr.2018.12.012 [138] Padhi A K, Nanjundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc, 1997, 144(4): 1188 doi: 10.1149/1.1837571 [139] Recham N, Rousse G, Sougrati M T, et al. Preparation and characterization of a stable FeSO4F-based framework for alkali ion insertion electrodes. Chem Mater, 2012, 24(22): 4363 doi: 10.1021/cm302428w [140] Chihara K, Katogi A, Kubota K, et al. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries. Chem Commun, 2017, 53(37): 5208 doi: 10.1039/C6CC10280H -




 下載:
下載: