-
摘要: 高功率快放型鋰離子電池是目前鋰離子電池領域研究的重點方向之一。為了獲得具有高功率密度的鋰離子電池,正極材料須具有較高的電壓和較高的電子與離子導電率,正極材料主要包括高電壓鈷酸鋰、鎳錳酸鋰和高電壓三元材料,負極材料包括碳系材料、鈦基材料和金屬氧化物材料,以及為提高首效和降低負極電位而采用的預嵌鋰方法,并對鋰離子電池電解液用鋰鹽、溶劑和添加劑進行了綜述。最終總結了功率密度測試方法,并對高功率鋰離子電池的研究進行展望。Abstract: High-power and fast-discharging lithium-ion battery, which can be used in smart power grids, rail transits, electromagnetic launch systems, aerospace systems, and so on, is one of the key research directions in the field of lithium-ion batteries and has attracted increasing attention in recent years. To obtain lithium-ion batteries with a high power density, the cathode materials should possess high voltage and high electronic/ionic conductivity, which can be realized by selecting high-voltage materials and modifying them to improve the voltage and reduce the battery’s internal resistance. Currently, the cathode materials of high-power lithium-ion batteries mainly include high-voltage LiCoO2, LiNi0.5Mn1.5O4, and Li(NiCoMn)O2 materials. Meanwhile, the anode materials include carbon- and Ti-based materials and metal oxides. This paper reviews the research on the modification of these materials, such as element doping and surface coating, which have gained considerable attention nowadays, as well as some new types of anode materials that exhibit excellent electrochemical properties. In terms of the negative electrode, the prelithiation process is one of the effective means to improve the power performance of a lithium-ion battery. This process’s significance is to compensate the consumption of Li+ and reduce the potential of the negative electrode to the working range for improving the platform voltage of the battery and improving the power density and energy density. This paper summarizes several commonly used prelithiation methods of the lithium-ion battery. Finally, the lithium salts, solvents, and additives for the electrolytes of lithium-ion batteries are introduced on the basis of their classification, properties, and performances. Several new types of lithium salts and additives are mentioned herein, such as lithium bis(fluorosulfonyl)imide, lithium bis(oxalate)borate, and tetramethylene sulfone. Furthermore, this paper summarizes several common power density test methods of lithium-ion batteries and prospects the research of high-power lithium-ion batteries. As a matter of fact, the power performance of lithium-ion batteries is gaining increasing attention and has truly achieved considerable improvement in recent years.
-
Key words:
- high-power lithium-ion batteries /
- cathode materials /
- anode materials /
- electrolytes /
- prelithiation /
- power density
-
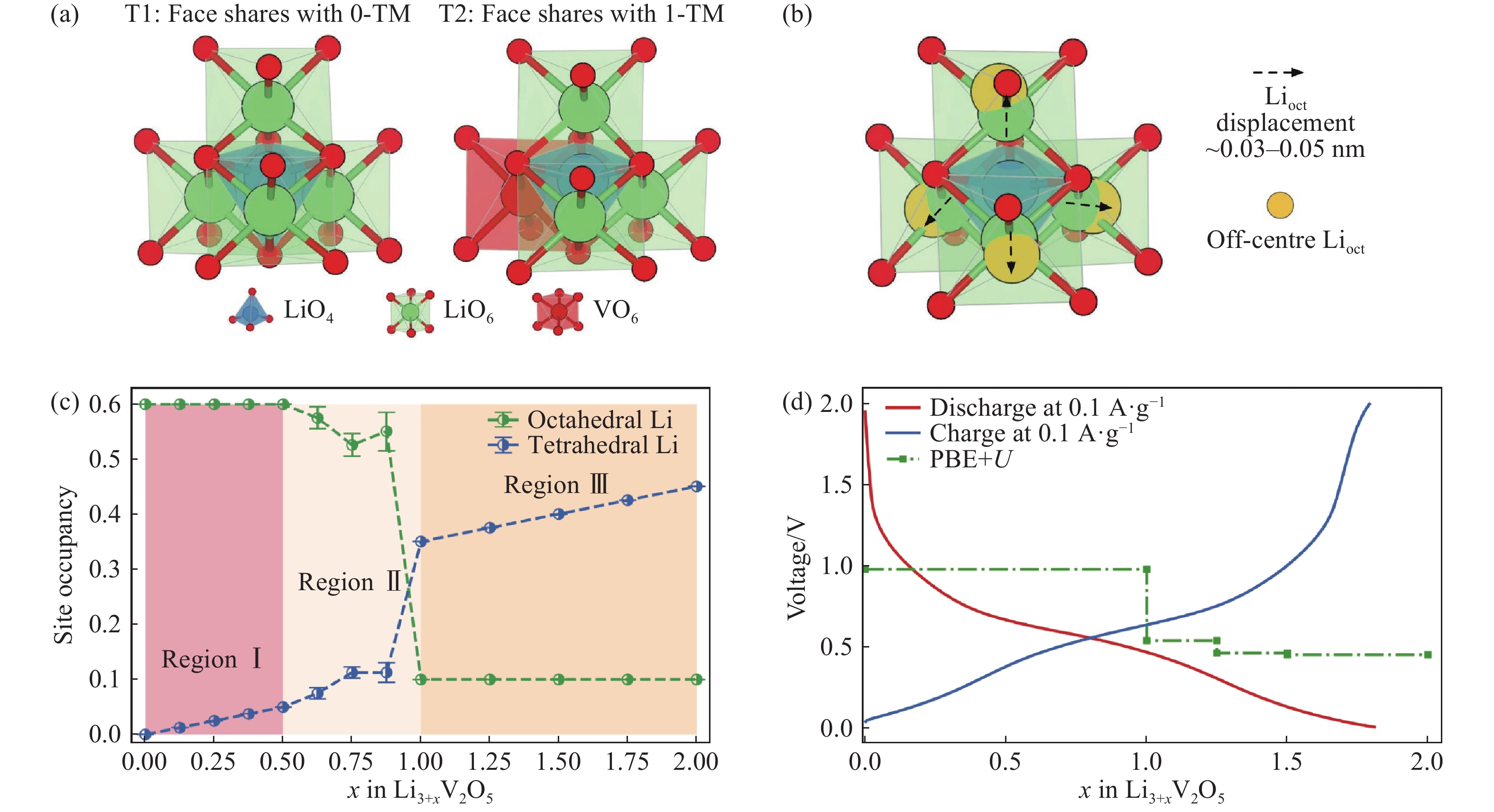
圖 4 密度泛函理論(Density functional theory, DFT)計算無序巖鹽結構Li3+xV2O5的Li位置占有率和電壓曲線[54]。(a)0-TM(T1)和1-TM(T2)四面體Li插入位點;(b)當Li插入0-TM(T1)位點時四個相鄰的LiO6八面體的偏心位移;(c)根據DFT計算,在插入Li+后四面體和八面體中Li位置占有率的演變;(d)相對于鋰電極的實驗電壓曲線和根據PBE+U泛函計算得出的的電壓曲線
Figure 4. DFT-calculated Li site occupancies and voltage profile for DRS-Li3+xV2O5[54]: (a) 0-TM (T1) and 1-TM (T2) tetrahedral Li insertion sites; (b) off-center displacements of four neighboring LiO6 octahedra upon Li insertion into the 0-TM (T1) site; (c) evolution of Li site occupancies in the tetrahedral and octahedral sites upon Li insertion determined via DFT calculations; (d) experimental and computational voltage profiles calculated using the PBE + U functional
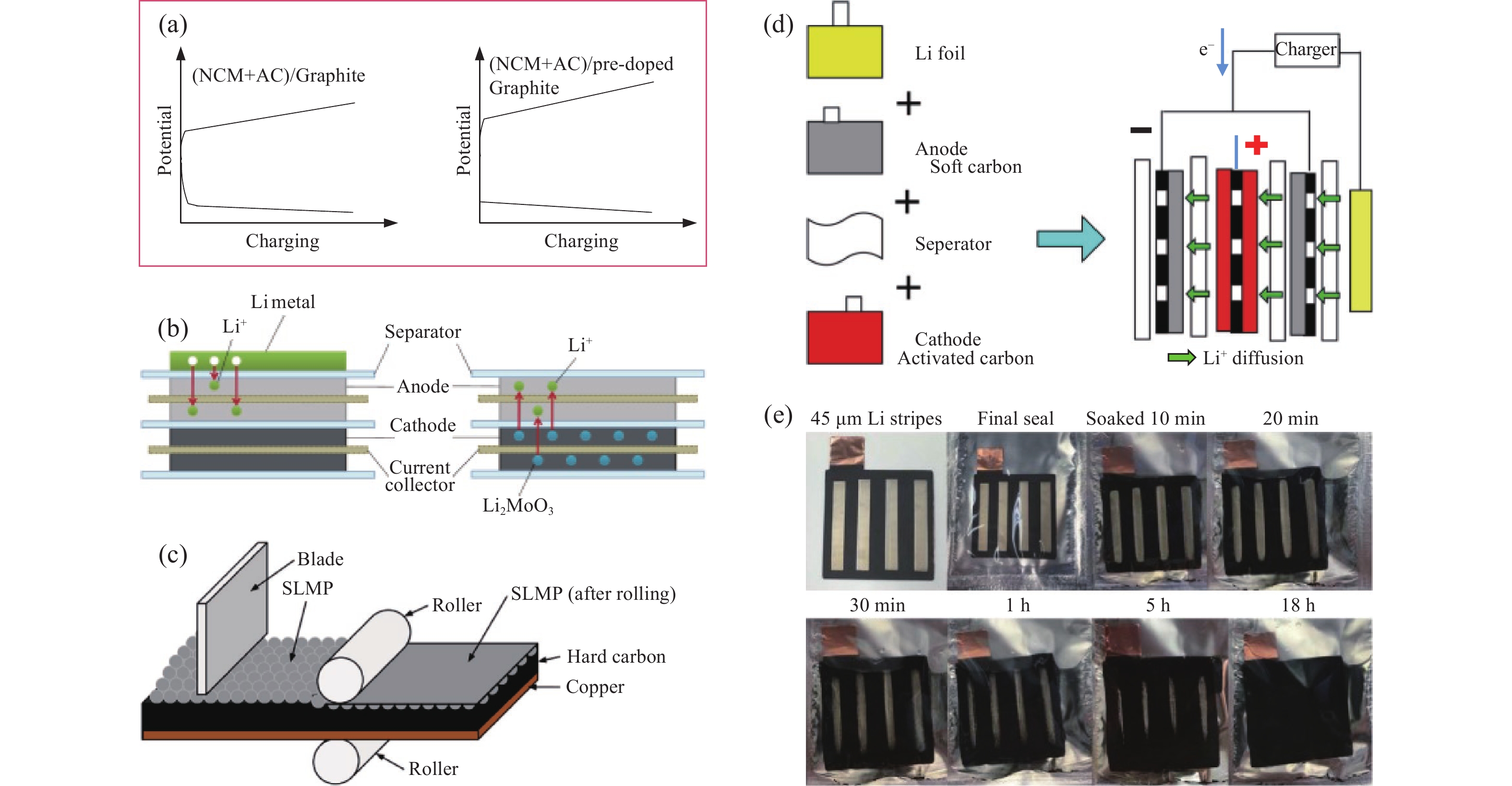
圖 6 高功率鋰離子電池預嵌鋰方法示意圖。(a)預嵌鋰效果圖[33];(b)負極摻雜鋰和正極摻雜鋰示意圖[82];(c)鈍化鋰粉(SLMP)方法預嵌鋰[83];(d)電化學方法預嵌鋰[84];(e)鋰金屬接觸方法預嵌鋰[85]
Figure 6. Schematic diagram of pre-lithiation method for high power LIBs: (a) potential changes before and after pre-lithiation [33];(b)pre-lithiation approaches of Li foil and cathode additives[82];(c) SLMP powder pre-lithiation method [83];(d)electrochemical pre-lithiation method [84];(e) Li metal contact prelithiation method [85]
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature, 2001, 414(6861): 359 doi: 10.1038/35104644 [2] Ministry of Industry and Information Technology, People’s Republic of China. GB/T 31486—2015 Electrical Performance Requirements and Test Methods for Traction Battery of Electric Vehicle. Beijing: China Standards Press, 2015中華人民共和國工業和信息化部. GB/T 31486—2015電動汽車用動力蓄電池電性能要求及試驗方法. 北京: 中國標準出版社, 2015 [3] Li C, Zhang X, Lv Z, et al. Scalable combustion synthesis of graphene-welded activated carbon for high-performance supercapacitors. Chem Eng J, 2021, 414: 128781 doi: 10.1016/j.cej.2021.128781 [4] Zhao S H, Wu F, Wang Z D, et al. Study on the different test methods for power density of power batteries. Acta Armamentarii, 2009, 30(6): 764趙淑紅, 吳鋒, 王子冬, 等. 動力電池功率密度性能測試評價方法的比較研究. 兵工學報, 2009, 30(6):764 [5] Zhang J, Liu J W, Liu Q B. Research on ultra-high power Li-ion battery. Chin J Power Sources, 2016, 40(5): 973張健, 劉建文, 劉全兵. 超高功率鋰離子電池研究. 電源技術, 2016, 40(5):973 [6] Zhang J B, Lian F, Gao X P, et al. Advance of lithium ion batteries: The 16th International Meeting on Lithium Batteries. Sci Sin Chimica, 2012, 42(8): 1252張劍波, 連芳, 高學平, 等. 鋰離子電池及材料發展前瞻——第16屆國際鋰電會議評述. 中國科學: 化學, 2012, 42(8): 1252 [7] Wang Y, Cao G Z. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv Mater, 2008, 20(12): 2251 doi: 10.1002/adma.200702242 [8] Zhang J N. Failure Analysis and Modification Research on High Voltage LiCoO2 [Dissertation]. Beijing: University of Chinese Academy of Sciences (Institute of Physics, CAS), 2018張杰男. 高電壓鈷酸鋰的失效分析與改性研究[學位論文]. 北京: 中國科學院大學(中國科學院物理研究所), 2018 [9] Gong Y, Zhang J N, Jiang L W, et al. In situ atomic-scale observation of electrochemical delithiation induced structure evolution of LiCoO2 cathode in a working all-solid-state battery. J Am Chem Soc, 2017, 139(12): 4274 doi: 10.1021/jacs.6b13344 [10] Zhang J N, Li Q H, Li Q, et al. Improved electrochemical performances of high voltage LiCoO2 with tungsten doping. Chin Phys B, 2018, 27(8): 088202 doi: 10.1088/1674-1056/27/8/088202 [11] Yang Q, Huang J, Li Y J, et al. Surface-protected LiCoO2 with ultrathin solid oxide electrolyte film for high-voltage lithium ion batteries and lithium polymer batteries. J Power Sources, 2018, 388: 65 doi: 10.1016/j.jpowsour.2018.03.076 [12] Tukamoto H, West A R. Electronic conductivity of LiCoO2 and its enhancement by magnesium doping. J Electrochem Soc, 1997, 144(9): 3164 doi: 10.1149/1.1837976 [13] Ceder G, Chiang Y M, Sadoway D R, et al. Identification of cathode materials for lithium batteries guided by first-principles calculations. Nature, 1998, 392(6677): 694 doi: 10.1038/33647 [14] Gopukumar S, Jeong Y, Kim K B. Synthesis and electrochemical performance of tetravalent doped LiCoO2 in lithium rechargeable cells. Solid State Ion, 2003, 159(3-4): 223 doi: 10.1016/S0167-2738(03)00081-X [15] Zhang J N, Li Q H, Ouyang C Y, et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat Energy, 2019, 4(7): 594 doi: 10.1038/s41560-019-0409-z [16] Morimoto H, Awano H, Terashima J, et al. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1+xAlxTi2?x(PO4)3 (x=0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J Power Sources, 2013, 240: 636 [17] Yano A, Shikano M, Ueda A, et al. LiCoO2 Degradation behavior in the high-voltage phase transition region and improved reversibility with surface coating. J Electrochem Soc, 2016, 164(1): A6116 [18] Jayasree S S, Nair S, Santhanagopalan D. Ultrathin TiO2 coating on LiCoO2 for improved electrochemical performance as Li–ion battery cathode. Chem Select, 2018, 3(10): 2763 [19] Zhang J N, Li Q H, Wang Y, et al. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interaction with Li anode. Energy Storage Mater, 2018, 14: 1 doi: 10.1016/j.ensm.2018.02.016 [20] Hong S K, Mho S I, Yeo I H, et al. Structural and electrochemical characteristics of morphology-controlled Li[Ni0.5Mn1.5]O4 cathodes. Electrochim Acta, 2015, 156: 29 doi: 10.1016/j.electacta.2015.01.027 [21] Fergus J W. Recent developments in cathode materials for lithium ion batteries. J Power Sources, 2010, 195(4): 939 doi: 10.1016/j.jpowsour.2009.08.089 [22] Chen L Q. Research progress in cathode materials of Li-ion battery. Battery Bimonthly, 2002, 32(S1), 6陳立泉. 鋰離子電池正極材料的研究進展. 電池, 2002, 32(S1): 6 [23] Jia G L, Jiao C M, Xue W J, et al. Improvement in electrochemical performance of calcined LiNi0.5Mn1.5O4/GO. Solid State Ion, 2016, 292: 15 doi: 10.1016/j.ssi.2016.05.003 [24] Fang X, Shen C F, Ge M Y, et al. High-power lithium ion batteries based on flexible and light-weight cathode of LiNi0.5Mn1.5O4/carbon nanotube film. Nano Energy, 2015, 12: 43 doi: 10.1016/j.nanoen.2014.11.052 [25] Chang Q, Wei A J, Li W, et al. Structural and electrochemical characteristics of Al2O3-modified LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries. Ceram Int, 2019, 45(4): 5100 doi: 10.1016/j.ceramint.2018.11.213 [26] Zhu R N, Zhang S J, Guo Q X, et al. More than just a protection layer: Inducing chemical interaction between Li3BO3 and LiNi0.5Mn1.5O4 to achieve stable high-rate cycling cathode materials. Electrochimical Acta, 2020, 342: 136074 doi: 10.1016/j.electacta.2020.136074 [27] Mou J R, Deng Y L, He L H, et al. Critical roles of semi-conductive LaFeO3 coating in enhancing cycling stability and rate capability of 5 V LiNi0.5Mn1.5O4 cathode materials. Electrochimical Acta, 2018, 260: 101 doi: 10.1016/j.electacta.2017.11.059 [28] Dong H, Zhang Y, Zhang S, et al. Improved high temperature performance of a spinel LiNi0.5Mn1.5O4 cathode for high-voltage lithium-ion batteries by surface modification of a flexible conductive nanolayer. ACS Omega, 2019, 4(1): 185 doi: 10.1021/acsomega.8b02571 [29] Jurng S, Heiskanen S K, Chandrasiri K, et al. Minimized metal dissolution from high-energy nickel cobalt manganese oxide cathodes with Al2O3 coating and its effects on electrolyte decomposition on graphite anodes. J Electrochem Soc, 2019, 166(13): A2721 doi: 10.1149/2.0101913jes [30] Park B C, Kim H B, Myung S T, et al. Improvement of structural and electrochemical properties of AlF3-coated Li[Ni1/3Co1/3Mn1/3]O2 cathode materials on high voltage region. J Power Sources, 2008, 178(2): 826 doi: 10.1016/j.jpowsour.2007.08.034 [31] Liu W J, Sun X Z, Zhang X, et al. Structural evolution of mesoporous graphene/LiNi1/3Co1/3Mn1/3O2 composite cathode for Li–ion battery. Rare Met, 2021, 40(3): 521 doi: 10.1007/s12598-020-01406-4 [32] Liu W J, Li C, Sun X Z, et al. Improvement of the high-rate capability of LiNi1/3Co1/3Mn1/3O2 cathode by adding highly electroconductive and mesoporous graphene. J Alloys Compd, 2018, 758: 206 doi: 10.1016/j.jallcom.2018.05.110 [33] Sun X Z, Zhang X, Wang K, et al. Lithium ion hybrid capacitor with high energy density. J Electrochem, 2017, 23(5): 586孫現眾, 張熊, 王凱, 等. 高能量密度的鋰離子混合型電容器. 電化學, 2017, 23(5):586 [34] Sun X Z, Zhang X, Huang B, et al. (LiNi0.5Co0.2Mn0.3O2 + AC)/graphite hybrid energy storage device with high specific energy and high rate capability. J Power Sources, 2013, 243: 361 doi: 10.1016/j.jpowsour.2013.06.038 [35] Sun X Z, Zhang X, Zhang H T, et al. High performance lithium-ion hybrid capacitors with pre-lithiated hard carbon anodes and bifunctional cathode electrodes. J Power Sources, 2014, 270: 318 doi: 10.1016/j.jpowsour.2014.07.146 [36] Du T, Liu Z E, Sun X Z, et al. Segmented bi-material cathodes to boost the lithium-ion battery-capacitors. J Power Sources, 2020, 478: 228994 doi: 10.1016/j.jpowsour.2020.228994 [37] Luo F, Chu G, Huang J, et al. Fundamental scientific aspects of lithium batteries(Ⅷ)—Anode electrode materials. Energy Storage Sci Technol, 2014, 3(2): 146羅飛, 褚賡, 黃杰, 等. 鋰離子電池基礎科學問題(Ⅷ)——負極材料. 儲能科學與技術, 2014, 3(2):146 [38] Li Z, Sun X Z, Liu W J, et al. A comparative study of pre-lithiated hard carbon and soft carbon as anodes for lithium-ion capacitors. J Electrochem, 2019, 25(1): 122 [39] Pan G H, Zhao Y B, Zhang K Z, et al. High power soft/hard carbon composite anode for rechargeable lithium-ion battery. Energy Storage Sci Technol, 2017, 6(1): 94潘廣宏, 趙永彬, 張開周, 等. 高功率鋰離子電池軟/硬復合碳負極材料. 儲能科學與技術, 2017, 6(1):94 [40] Zhang H T, Sun X Z, Zhang X, et al. High-capacity nanocarbon anodes for lithium-ion batteries. J Alloys Compd, 2015, 622: 783 doi: 10.1016/j.jallcom.2014.10.188 [41] Zhang H T, Wang K, Zhang X, et al. Self-generating graphene and porous nanocarbon composites for capacitive energy storage. J Mater Chem A, 2015, 3(21): 11277 doi: 10.1039/C5TA01783A [42] Lu Y, Zhang H, Cao G P, et al. The progress in improving rate performance of anode material Li4Ti5O12. Battery Bimon, 2010, 40(3): 164呂巖, 張浩, 曹高萍, 等. 提高負極材料Li4Ti5O12倍率性能的進展. 電池, 2010, 40(3):164 [43] Qian D L, Gu Y J, Chen Y B, et al. Ultra-high specific capacity of Cr3+-doped Li4Ti5O12 at 1.55 V as anode material for lithium-ion batteries. Mater Lett, 2019, 238: 102 [44] Han Y H. Preparation and Electrochemical Performance of Lithium Titanate as Anode Materials for Li-Ion Batteries [Dissertation]. Yantai: Yantai University, 2019韓葉虎. 鋰離子電池負極材料鈦酸鋰改性制備及電化學性能研究[學位論文]. 煙臺: 煙臺大學, 2019 [45] Yan G L, Xu X R, Zhang W T, et al. Preparation and electrochemical performance of P5+-doped Li4Ti5O12 as anode material for lithium-ion batteries. Nanotechnology, 2020, 31(20): 205402 doi: 10.1088/1361-6528/ab7047 [46] Yan G L. Preparation and Performance of Lithium Titanate Anode Materials for Lithium-Ion Batteries [Dissertation]. Chengdu: Chengdu University of Technology, 2020嚴桂林. 鋰離子電池用鈦酸鋰負極材料的制備與性能研究[學位論文]. 成都: 成都理工大學, 2020 [47] Zhao X R, An Q, Ma X D, et al. Research progress of metal oxides as anode materials for lithium ion capacitors. Energy Storage Sci Technol, 2018, 7(4): 555趙興茹, 安琪, 馬向東, 等. 金屬氧化物作為鋰離子電容器負極的研究進展. 儲能科學與技術, 2018, 7(4):555 [48] Li C, Zhang X, Wang K, et al. A 29.3 Wh kg?1 and 6 kW kg?1 pouch-type lithium-ion capacitor based on SiOx/graphite composite anode. J Power Sources, 2019, 414: 293 [49] Sun X Z, Geng L B, Yi S, et al. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J Power Sources, 2021, 499: 229936 doi: 10.1016/j.jpowsour.2021.229936 [50] Lv P P, Zhao H L, Li Z L, et al. Citrate-nitrate gel combustion synthesis of micro/nanostructured SiOx/C composite as high-performance lithium-ion battery anode. Solid State Ion, 2019, 340: 115024 doi: 10.1016/j.ssi.2019.115024 [51] Lu X X, Mao Q N, Chen Y F, et al. A novel oxygen vacancy introduced microstructural reconstruction of SnO2-graphene nanocomposite: Demonstration of enhanced electrochemical performance for sodium storage. Electrochimica Acta, 2018, 282: 351 doi: 10.1016/j.electacta.2018.06.069 [52] Zhao X R, Zhang X, Li C, et al. High-performance lithium-ion capacitors based on CoO-graphene composite anode and holey carbon nanolayer cathode. ACS Sustainable Chem Eng, 2019, 7(13): 11275 doi: 10.1021/acssuschemeng.9b00641 [53] Yuan T Z, Jiang Y Z, Sun W P, et al. Ever-increasing pseudocapacitance in RGO-MnO-RGO sandwich nanostructures for ultrahigh-rate lithium storage. Adv Funct Mater, 2016, 26(13): 2198 doi: 10.1002/adfm.201504849 [54] Liu H D, Zhu Z Y, Yan Q Z, et al. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature, 2020, 585(7823): 63 doi: 10.1038/s41586-020-2637-6 [55] Liu W J, Zhang X, Li C, et al. Carbon-coated Li3VO4 with optimized structure as high capacity anode material for lithium-ion capacitors. Chin Chem Lett, 2020, 31(9): 2225 doi: 10.1016/j.cclet.2019.11.015 [56] Zhang S J, Li C, Zhang X, et al. High performance lithium-ion hybrid capacitors employing Fe3O4-graphene composite anode and activated carbon cathode. ACS Appl Mater Interfaces, 2017, 9(20): 17136 doi: 10.1021/acsami.7b03452 [57] Qu X L, Ren Z H, Yang Y X, et al. Solid-state sintering strategy for simultaneous nanosizing and surface coating of iron oxides as high-capacity anodes for long life Li-ion batteries. ACS Appl Energy Mater, 2018, 1(11): 6330 doi: 10.1021/acsaem.8b01308 [58] Qin L, Liu Y, Xu S Y, et al. In-plane assembled single-crystalline T-Nb2O5 nanorods derived from few-layered Nb2CTx MXene nanosheets for advanced Li-ion capacitors. Small Methods, 2020, 4(12): 2000630 doi: 10.1002/smtd.202000630 [59] Qu X L, Zhang X, Wu Y J, et al. An eggshell-structured N-doped silicon composite anode with high anti-pulverization and favorable electronic conductivity. J Power Sources, 2019, 443: 227265 doi: 10.1016/j.jpowsour.2019.227265 [60] Yang Y X, Ni C L, Gao M X, et al. Dispersion-strengthened microparticle silicon composite with high anti-pulverization capability for Li-ion batteries. Energy Storage Mater, 2018, 14: 279 doi: 10.1016/j.ensm.2018.04.008 [61] An Y B, Chen S, Zou M M, et al. Improving anode performances of lithium-ion capacitors employing carbon–Si composites. Rare Met, 2019, 38(12): 1113 doi: 10.1007/s12598-019-01328-w [62] Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev, 2004, 104(10): 4303 doi: 10.1021/cr030203g [63] Zhuang Q C, Wu S, Liu W Y, et al. The research of organic electrolyte solutions for Li-ion batteries. Electrochemistry, 2001, 7(4): 403莊全超, 武山, 劉文元, 等. 鋰離子電池有機電解液研究. 電化學, 2001, 7(4):403 [64] Liu Y L, Wu J Y, Li H. Fundamental scientific aspects of lithium ion batteries (Ⅸ)—Nonaqueous electrolyte materials. Energy Storage Sci Technol, 2014(3): 262劉亞利, 吳嬌楊, 李泓. 鋰離子電池基礎科學問題(Ⅸ)—非水液體電解質材料. 儲能科學與技術, 2014(3):262 [65] Mu D Y, Liu Y L, Dai C S. Research progress in liquid organic electrolyte for Li-ion battery. Battery Bimon, 2019, 49(1): 68穆德穎, 劉元龍, 戴長松. 鋰離子電池液態有機電解液的研究進展. 電池, 2019, 49(1):68 [66] Zhang X Y, Zhou Y, Deng X Y, et al. Progress in LiBF4-based liquid electrolytes for Li-ion batteries. Chemistry, 2007, 70(12): 929張昕岳, 周園, 鄧小宇, 等. 鋰離子電池LiBF4基液體電解質研究進展. 化學通報, 2007, 70(12):929 [67] Dong Q Y, Guo F, Cheng Z J, et al. Insights into the dual role of lithium difluoro(oxalato)borate additive in improving the electrochemical performance of NMC811||Graphite cells. ACS Appl Energy Mater, 2020, 3(1): 695 doi: 10.1021/acsaem.9b01894 [68] Ehteshami N, Ibing L, Stolz L, et al. Ethylene carbonate-free electrolytes for Li-ion battery: Study of the solid electrolyte interphases formed on graphite anodes. J Power Sources, 2020, 451: 227804 doi: 10.1016/j.jpowsour.2020.227804 [69] Dougassa Y R, Jacquemin J, El Ouatani L, et al. Viscosity and carbon dioxide solubility for LiPF6, LiTFSI, and LiFAP in alkyl carbonates: lithium salt nature and concentration effect. J Phys Chem B, 2014, 118(14): 3973 doi: 10.1021/jp500063c [70] Li M, Qiu J Y, Yu Z B, et al. New use of conducting salts in electrolytes of high power Li ion batteries. Chin J Power Sources, 2015, 39(1): 191李萌, 邱景義, 余仲寶, 等. 高功率鋰離子電池電解液中導電鋰鹽的新應用. 電源技術, 2015, 39(1):191 [71] Han H B, Zhou S S, Zhang D J, et al. Lithium bis(fluorosulfonyl)imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. J Power Sources, 2011, 196(7): 3623 doi: 10.1016/j.jpowsour.2010.12.040 [72] Balakrishnan P G, Ramesh R, Prem Kumar T. Safety mechanisms in lithium-ion batteries. J Power Sources, 2006, 155(2): 401 doi: 10.1016/j.jpowsour.2005.12.002 [73] Yun J J, Zhang L, Qu Q T, et al. A binary cyclic carbonates-based electrolyte containing propylene carbonate and trifluoropropylene carbonate for 5 V lithium-ion batteries. Electrochim Acta, 2015, 167: 151 doi: 10.1016/j.electacta.2015.03.159 [74] Wu F, Zhou H, Bai Y, et al. Toward 5 V Li-ion batteries: Quantum chemical calculation and electrochemical characterization of sulfone-based high-voltage electrolytes. ACS Appl Mater Interfaces, 2015, 7(27): 15098 doi: 10.1021/acsami.5b04477 [75] Chen R J, Zhu L, Wu F, et al. Investigation of a novel ternary electrolyte based on dimethyl sulfite and lithium difluoromono(oxalato)borate for lithium ion batteries. J Power Sources, 2014, 245: 730 doi: 10.1016/j.jpowsour.2013.06.132 [76] Ming J, Cao Z, Wu Y Q, et al. New insight on the role of electrolyte additives in rechargeable lithium ion batteries. ACS Energy Letters, 2019, 4(11): 2613 doi: 10.1021/acsenergylett.9b01441 [77] Li F F, Chen S M. Research progress on electrolyte additives for high voltage lithium-ion batteries. Energy Storage Sci Technol, 2016, 5(4): 436李放放, 陳仕謀. 高壓鋰離子電池電解液添加劑研究進展. 儲能科學與技術, 2016, 5(4):436 [78] Zhou M J, Qin C Y, Liu Z, et al. Enhanced high voltage cyclability of LiCoO2 cathode by adopting poly[bis-(ethoxyethoxyethoxy)phosphazene] with flame-retardant property as an electrolyte additive for lithium-ion batteries. Appl Surf Sci, 2017, 403: 260 doi: 10.1016/j.apsusc.2017.01.189 [79] Lee Y M, Nam K M, Hwang E H, et al. Interfacial origin of performance improvement and fade for 4.6 V LiNi0. 5Co0.2Mn0.3O2 battery cathodes. J Phys Chem C, 2014, 118(20): 10631 [80] Zuo X X, Fan C J, Liu J S, et al. Effect of tris(trimethylsilyl)borate on the high voltage capacity retention of LiNi0.5Co0.2Mn0.3O2/graphite cells. J Power Sources, 2013, 229: 308 doi: 10.1016/j.jpowsour.2012.12.056 [81] Xu H W, Shi J L, Hu G S, et al. Hybrid electrolytes incorporated with dandelion-like silane Al2O3 nanoparticles for high-safety high-voltage lithium ion batteries. J Power Sources, 2018, 391: 113 doi: 10.1016/j.jpowsour.2018.04.060 [82] Park M S, Lim Y G, Kim J H, et al. A novel lithium-doping approach for an advanced lithium ion capacitor. Adv Energy Mater, 2011, 1(6): 1002 doi: 10.1002/aenm.201100270 [83] Cao W J, Shih J, Zheng J P, et al. Development and characterization of Li-ion capacitor pouch cells. J Power Sources, 2014, 257: 388 doi: 10.1016/j.jpowsour.2014.01.087 [84] Sun X Z, An Y B, Geng L B, et al. Leakage current and self-discharge in lithium-ion capacitor. J Electroanal Chem, 2019, 850: 113386 doi: 10.1016/j.jelechem.2019.113386 [85] Cao W J, Luo J F, Yan J, et al. High performance Li-ion capacitor laminate cells based on hard carbon/lithium stripes negative electrodes. J Electrochem Soc, 2016, 164(2): A93 [86] Sun C K, Zhang X, Li C, et al. Recent advances in prelithiation materials and approaches for lithium-ion batteries and capacitors. Energy Storage Mater, 2020, 32: 497 doi: 10.1016/j.ensm.2020.07.009 [87] Je?owski P, Fic K, Crosnier O, et al. Lithium rhenium(vii) oxide as a novel material for graphite pre-lithiation in high performance lithium-ion capacitors. J Mater Chem A, 2016, 4(32): 12609 doi: 10.1039/C6TA03810G [88] Guo Y T, Li X H, Wang Z X, et al. Bifunctional Li6CoO4 serving as prelithiation reagent and pseudocapacitive electrode for lithium ion capacitors. J Energy Chem, 2020, 47: 38 doi: 10.1016/j.jechem.2019.11.003 [89] Zhang S S. Eliminating pre-lithiation step for making high energy density hybrid Li-ion capacitor. J Power Sources, 2017, 343: 322 doi: 10.1016/j.jpowsour.2017.01.061 [90] Park M S, Lim Y G, Hwang S M, et al. Scalable Integration of Li5FeO4 towards robust, high‐performance lithium‐ion hybrid capacitors. ChemSusChem, 2014, 7(11): 3138 doi: 10.1002/cssc.201402397 [91] Arnaiz M, Shanmukaraj D, Carriazo D, et al. A transversal low-cost pre-metallation strategy enabling ultrafast and stable metal ion capacitor technologies. Energy Environ Sci, 2020, 13(8): 2441 doi: 10.1039/D0EE00351D [92] Hwang S W, Yoon W Y. Effect of Li powder-coated separator on irreversible behavior of SiOx-C anode in lithium-ion batteries. J Electrochem Soc, 2014, 161(10): A1753 doi: 10.1149/2.0031412jes [93] Ai G, Wang Z H, Zhao H, et al. Scalable process for application of stabilized lithium metal powder in Li-ion batteries. J Power Sources, 2016, 309: 33 doi: 10.1016/j.jpowsour.2016.01.061 [94] Je?owski P, Crosnier O, Deunf E, et al. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat Mater, 2018, 17(2): 167 doi: 10.1038/nmat5029 [95] An F Q, He D L, Pang Z, et al. Preparation of silicon /graphite /carbon composites with fiber carbon and their application in lithium-ion batteries. Chin J Eng, 2019, 41(10): 1307安富強, 何冬林, 龐錚, 等. 具有微米纖維碳的硅/石墨/碳復合材料的制備及在鋰離子電池中的應用. 工程科學學報, 2019, 41(10):1307 [96] Wang S J, Liu Q G. Carbonized production of phenolic aldehyde as the electrode materials of lithium ion batteries (ii)——analyzed of the charge and discharge performance of the lithium ion cells. Chin J Eng, 2000, 22(6): 533汪樹軍, 劉慶國. 酚醛樹脂碳化產物作為鋰離子電池碳電極材料(Ⅱ)——鋰離子電池充放電性能測試. 工程科學學報, 2000, 22(6):533 [97] Zhang X H, Sun X Z, Zhang X, et al. Prospect of lithium-ion capacitor application in new energy field. Adv Technol Electr Eng Energy, 2020, 39(11): 48張曉虎, 孫現眾, 張熊, 等. 鋰離子電容器在新能源領域應用展望. 電工電能新技術, 2020, 39(11):48 [98] Ajuria J, Aguesse F. An ultrafast battery performing as a supercapacitor: Electrode tuning for high power performance. Electrochimica Acta, 2020, 334: 135587 doi: 10.1016/j.electacta.2019.135587 -




 下載:
下載: