-
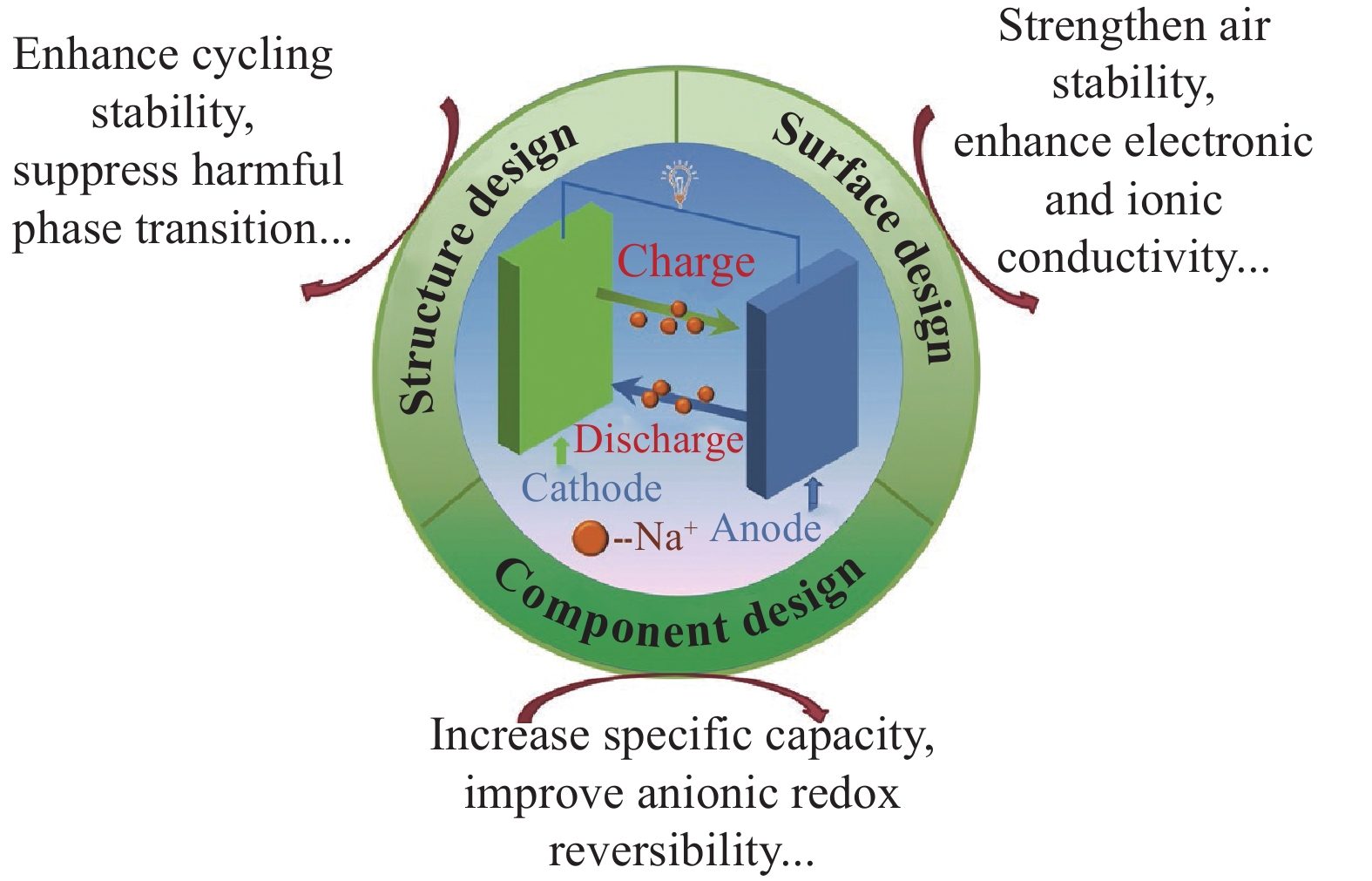
摘要: 鈉離子電池憑借資源和成本優勢在大規模儲能和低速電動車領域展現出極大應用前景。層狀氧化物理論容量較高且易于合成,是目前最具應用潛力的鈉離子電池正極材料之一。如何改善層狀氧化物正極材料的循環穩定性并提升其能量密度是當前的科學前沿問題。首先,綜述了層狀氧化物正極材料的幾種典型改性方法,從組分設計的角度,探討了不同摻雜元素、不同摻雜位點對材料容量和循環壽命的影響,闡述了利用陰離子反應提供額外容量的基本原理,概述了提高陰離子氧化還原可逆性的摻雜策略;從結構設計的角度,介紹了復合相材料的制備、微觀結構的設計和調控等方向的最新進展;從表面設計的角度,討論了金屬氧化物、磷酸鹽等作為包覆層對改善材料穩定性和倍率性能的作用機制。最后,總結了層狀氧化物儲鈉正極材料現階段面臨的挑戰,并對其未來的發展方向進行了展望,提出了新的研究思路。Abstract: Driven by the national strategic goal of “emission peak and carbon neutrality”, developing grid-scale energy storage systems (ESSs) for high-efficiency utilization of renewable clean energy is of great importance and urgency. Currently, lithium-ion batteries (LIBs) are being widely used in portable electronics and electric vehicles markets due to their high energy density and long cycling life. Nevertheless, the ever-increasing price and uneven distribution of lithium resources limit the further applications of LIBs for large-scale ESS. Recently, sodium-ion batteries (SIBs) have gained tremendous attention as promising large-scale energy storage devices and low-speed electric vehicle power sources, owing to the low-cost and abundant sodium reserves. However, the larger size and heavier mass of Na+ than those of Li+ commonly lead to sluggish reaction kinetics, severe volume expansion, and the undesirable structural failure of electrode materials upon charge/discharge, which hinder the commercial value of SIBs. Leveraging high-performance cathode materials is expected to boost the development of SIBs because cathodes largely determine the cost and electrochemical performance of batteries. Among the reported cathode candidates, layered oxide materials hold great potential due to their high capacity and a facile synthesis process; however, these materials face some challenges such as low capacity retention and poor air stability. Recently, exploring appropriate methods to strengthen the structural stability and further enhance the energy density of layered oxides has become an emerging research hotspot. In this regard, various strategies, such as element composition and relative content manipulation and microstructure and surface/interface modulation, have been proposed. In this review, typical modification methods for improving the Na-storage performance of layered oxide cathodes are comprehensively summarized. From the perspective of component design, the effects of different doping elements and doping sites on the capacity and cycling life are discussed. In addition, the basic principle of anionic redox reaction to offer extra capacity is elucidated, and the doping strategies for enhancing the anionic redox reversibility are outlined. From the perspective of structure design, the recent progresses on the preparation of composite phase materials and microstructures design are introduced. From the perspective of surface design, the functional mechanism of metal oxides and phosphates as coating layers to improve the structural stability and rate performance is explored. Finally, the challenging issues facing layered sodium oxide cathodes and possible remedies in the future are discussed. We believe that this review will shed light on the development of advanced layered oxide cathode materials for SIBs.
-
Key words:
- sodium-ion batteries /
- cathode materials /
- layered oxides /
- modification methods /
- anionic redox
-
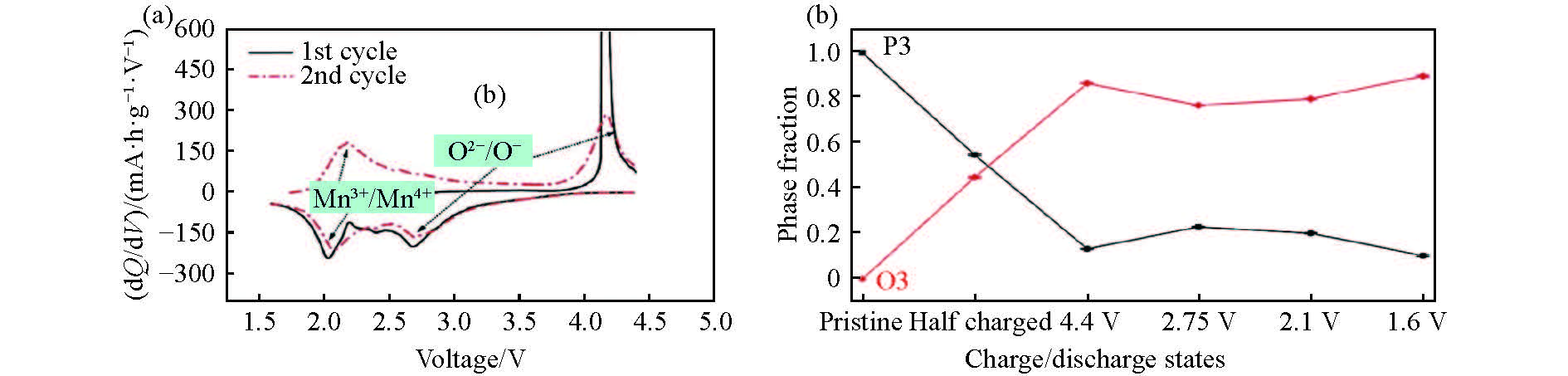
圖 7 P3-Na2/3Mg1/3Mn2/3O2 (a)在1.6~4.4 V(vs Na+/Na)電壓范圍內第一周和第二周的充放電dQ/dV圖(電流密度為9.7 mA·g?1);(b)不同充放電狀態下P3相和O3相的占比分數(采用Rietveld精修方法)[59]
Figure 7. (a) dQ/dV plots of P3-Na2/3Mg1/3Mn2/3O2 in the first and second cycles at 9.7 mA·g?1 from 1.6 V to 4.4 V (vs Na+/Na); (b) refined phase fractions of the P3 and O3 phases (using the Rietveld refinement method) at various charge/discharge states of Na2/3Mg1/3Mn2/3O2[59]
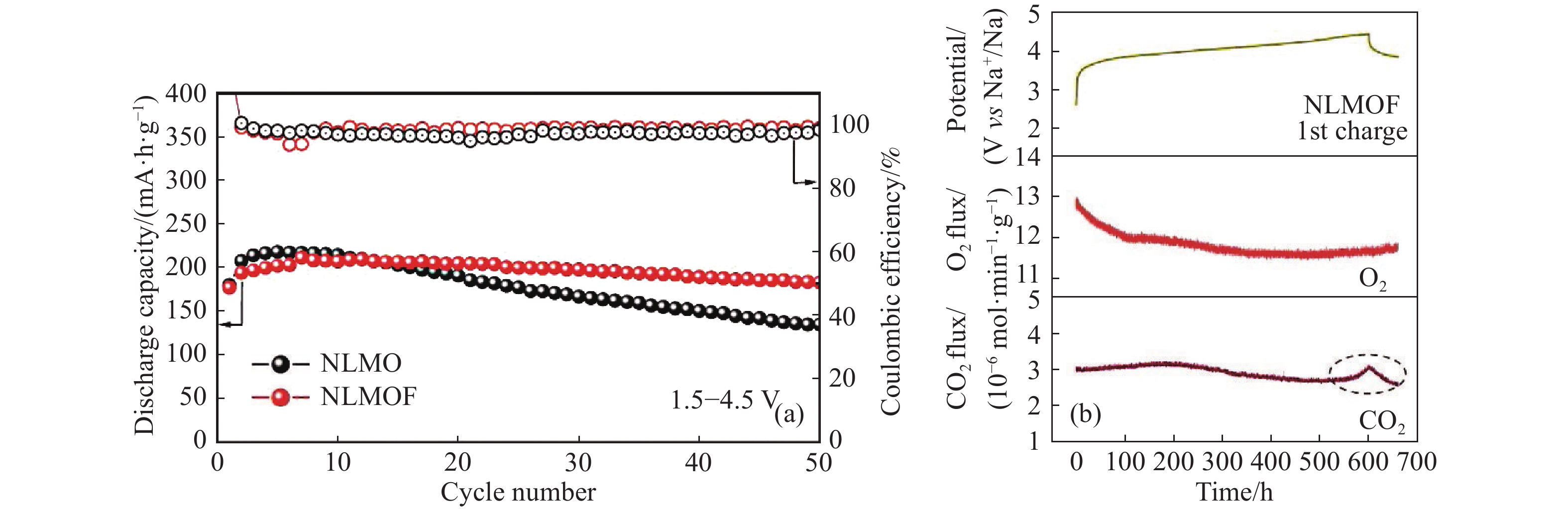
圖 8 (a)P2-Na0.65Li0.22Mn0.78O1.99F0.01與P2-Na0.66Li0.22Mn0.78O2的循環性能對比(電流密度為10 mA·g?1);(b)P2-NLMOF首次充電過程的原位差示電化學質譜(DEMS)氣體釋放結果[67]
Figure 8. (a) Comparison of the cycling performance at 10 mA·g?1 between P2-Na0.65Li0.22Mn0.78O1.99F0.01 and P2-Na0.66Li0.22Mn0.78O2; (b) operando DEMS results of gas evolution during the first charge process of P2-NLMOF[67]
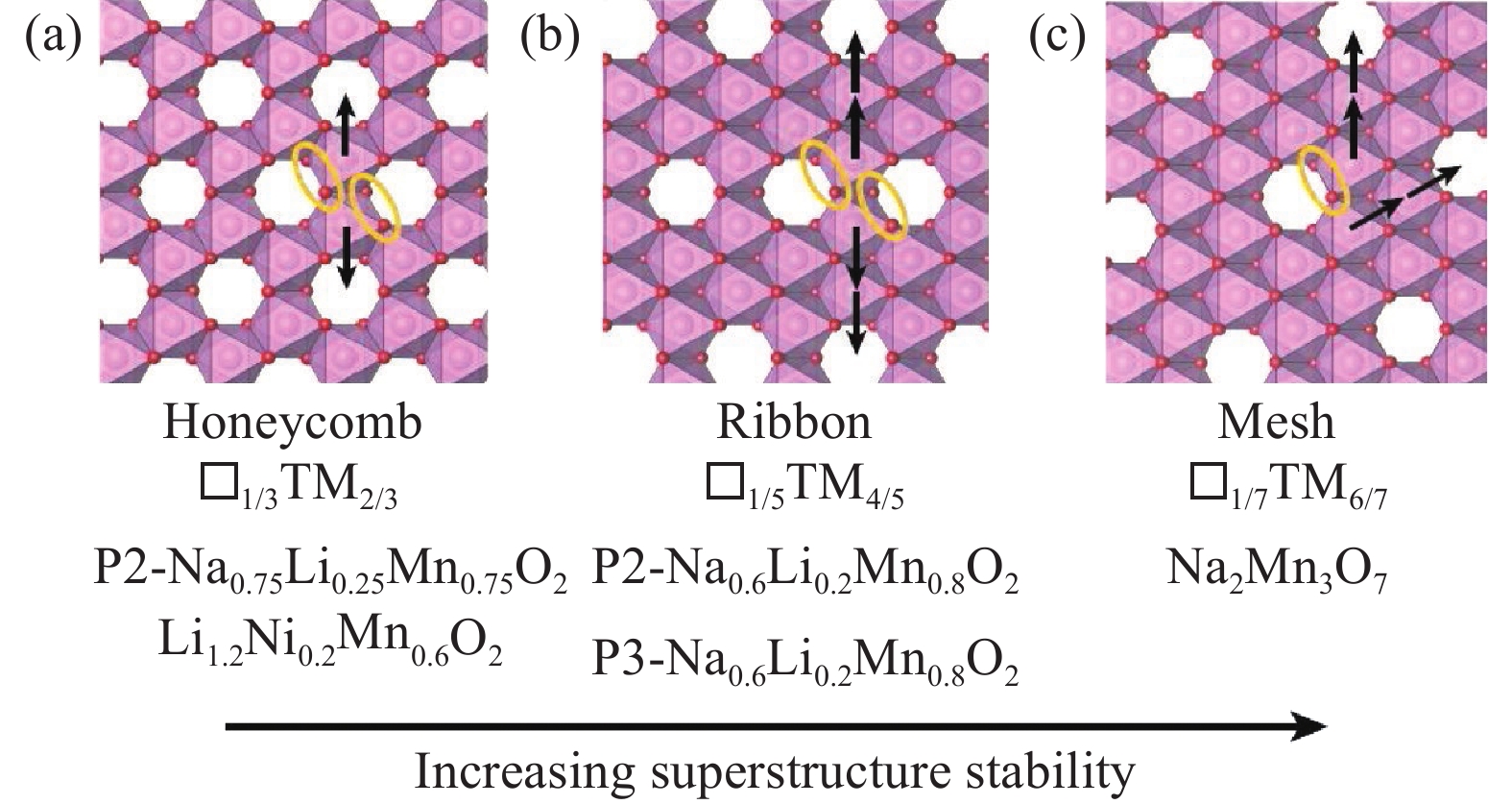
圖 10 充電狀態下在具有(a)蜂窩狀,(b)帶狀,(c)網狀超結構的TM層中形成O2分子(橙色橢圓形)時面內Mn3+遷移路徑(箭頭所示),□代表過渡金屬層的空位[73]
Figure 10. In-plane Mn3+ migration paths (shown by the arrow) in the (a) honeycomb, (b) ribbon, and (c) mesh superstructures in TM layers when O2 molecules (orange ellipse) are formed during charging, □ represents the vacancies in the TM layer [73]
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Vaalma C, Buchholz D, Weil M, et al. A cost and resource analysis of sodium-ion batteries. Nat Rev Mater, 2018, 3: 18013 doi: 10.1038/natrevmats.2018.13 [2] An F Q, Zhao H L, Cheng Z, et al. Development status and research progress of power battery for pure electric vehicles. Chin J Eng, 2019, 41(1): 22安富強, 趙洪量, 程志, 等. 純電動車用鋰離子電池發展現狀與研究進展. 工程科學學報, 2019, 41(1):22 [3] Choi J W, Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater, 2016, 1: 16013 doi: 10.1038/natrevmats.2016.13 [4] Guo J Z, Wan F, Wu X L, et al. Sodium-ion batteries: Work mechanism and the research progress of key electrode materials. J Mol Sci, 2016, 32(4): 265郭晉芝, 萬放, 吳興隆, 等. 鈉離子電池工作原理及關鍵電極材料研究進展. 分子科學學報, 2016, 32(4):265 [5] Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries. Chem Rev, 2014, 114(23): 11636 doi: 10.1021/cr500192f [6] Liu Y C, Chen C C, Zhang N, et al. Research and application of key materials for sodium-ion batteries. J Electrochem, 2016, 22(5): 437劉永暢, 陳程成, 張寧, 等. 鈉離子電池關鍵材料研究及應用進展. 電化學, 2016, 22(5):437 [7] Slater M D, Kim D, Lee E, et al. Sodium-ion batteries. Adv Funct Mater, 2013, 23(8): 947 doi: 10.1002/adfm.201200691 [8] Zuo W H, Qiu J M, Liu X S, et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres. Nat Commun, 2020, 11: 3544 doi: 10.1038/s41467-020-17290-6 [9] Cao Y L. The opportunities and challenges of sodium ion battery. Energy Storage Sci Technol, 2020, 9(3): 757曹余良. 鈉離子電池機遇與挑戰. 儲能科學與技術, 2020, 9(3):757 [10] Liu Q N, Hu Z, Chen M Z, et al. Recent progress of layered transition metal oxide cathodes for sodium-ion batteries. Small, 2019, 15(32): 1805381 doi: 10.1002/smll.201805381 [11] Shen X, Zhao J M, Li Y Q, et al. Controlled synthesis of Na3(VOPO4)2F cathodes with an ultralong cycling performance. ACS Appl Energy Mater, 2019, 2(10): 7474 doi: 10.1021/acsaem.9b01458 [12] Jin T, Li H X, Zhu K J, et al. Polyanion-type cathode materials for sodium-ion batteries. Chem Soc Rev, 2020, 49(8): 2342 doi: 10.1039/C9CS00846B [13] Chen S Q, Wu C, Shen L F, et al. Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries. Adv Mater, 2017, 29(48): 1700431 doi: 10.1002/adma.201700431 [14] Lee M, Hong J, Lopez J, et al. High-performance sodium–organic battery by realizing four-sodium storage in disodium rhodizonate. Nat Energy, 2017, 2(11): 861 doi: 10.1038/s41560-017-0014-y [15] Mao Y J, Chen Y T, Qin J, et al. Capacitance controlled, hierarchical porous 3D ultra-thin carbon networks reinforced Prussian blue for high performance Na-ion battery cathode. Nano Energy, 2019, 58: 192 doi: 10.1016/j.nanoen.2019.01.048 [16] Shao M M, Wang B, Liu M C, et al. A high-voltage and cycle stable aqueous rechargeable Na-ion battery based on Na2Zn3[Fe(CN)6]2-NaTi2(PO4)3 intercalation chemistry. ACS Appl Energy Mater, 2019, 2(8): 5809 doi: 10.1021/acsaem.9b00935 [17] Delmas C, Fouassier C, Hagenmuller P. Structural classification and properties of the layered oxides. Phys B+C, 1980, 99(1-4): 81 doi: 10.1016/0378-4363(80)90214-4 [18] Wang P F, You Y, Yin Y X, et al. Suppressing the P2-O2 phase transition of Na0.67Mn0.67Ni0.33O2 by magnesium substitution for improved sodium-ion batteries. Angew Chem Int Ed, 2016, 128(26): 7571 doi: 10.1002/ange.201602202 [19] Fang Y J, Yu X Y, Lou X W. A practical high-energy cathode for sodium-ion batteries based on uniform P2-Na0.7CoO2 microspheres. Angew Chem Int Ed, 2017, 56(21): 5801 doi: 10.1002/anie.201702024 [20] Zhou P F, Che Z N, Ma F T, et al. Designing water/air-stable P2-layered cathodes with delayed P2-O2 phase transition by composition and structure engineering for sodium-ion batteries at high voltage. Chem Eng J, 2021, 420: 127667 doi: 10.1016/j.cej.2020.127667 [21] Sun Y K. Direction for commercialization of O3-Type layered cathodes for sodium-ion batteries. ACS Energy Lett, 2020, 5(4): 1278 doi: 10.1021/acsenergylett.0c00597 [22] Zhang C, Gao R, Zheng L R, et al. New insights into the roles of Mg in improving the rate capability and cycling stability of O3-NaMn0.48Ni0.2Fe0.3Mg0.02O2 for sodium-ion batteries. ACS Appl Mater Interfaces, 2018, 10(13): 10819 doi: 10.1021/acsami.7b18226 [23] Liu J T, Kan W H, Ling C D. Insights into the high voltage layered oxide cathode materials in sodium-ion batteries: Structural evolution and anion redox. J Power Sources, 2021, 481: 229139 doi: 10.1016/j.jpowsour.2020.229139 [24] Gao R M, Zheng Z J, Wang P F, et al. Recent advances and prospects of layered transition metal oxide cathodes for sodium-ion batteries. Energy Storage Mater, 2020, 30: 9 doi: 10.1016/j.ensm.2020.04.040 [25] Deng J Q, Luo W B, Lu X, et al. High energy density sodium-ion battery with industrially feasible and air-stable O3-Type layered oxide cathode. Adv Energy Mater, 2018, 8(5): 1701610 doi: 10.1002/aenm.201701610 [26] Jiang Y L, Zou G Q, Hou H S, et al. Composition engineering boosts voltage windows for advanced sodium-ion batteries. ACS Nano, 2019, 13(9): 10787 doi: 10.1021/acsnano.9b05614 [27] Jin J T, Liu Y C, Pang X L, et al. A comprehensive understanding of the anionic redox chemistry in layered oxide cathodes for sodium-ion batteries. Sci China Chem, 2021, 64(3): 385 doi: 10.1007/s11426-020-9897-8 [28] Whittingham M S. Lithium batteries and cathode materials. Chem Rev, 2004, 104(10): 4271 doi: 10.1021/cr020731c [29] Rai A K, Anh L T, Gim J, et al. Electrochemical properties of NaxCoO2 (x~0.71) cathode for rechargeable sodium-ion batteries. Ceram Int, 2014, 40(1): 2411 [30] Lei Y C, Li X, Liu L, et al. Synthesis and stoichiometry of different layered sodium cobalt oxides. Chem Mater, 2014, 26(18): 5288 doi: 10.1021/cm5021788 [31] Yabuuchi N, Yoshida H, Komaba S. Crystal structures and electrode performance of alpha-NaFeO2 for rechargeable sodium batteries. Electrochemistry, 2012, 80(10): 716 doi: 10.5796/electrochemistry.80.716 [32] Guo S H, Yu H J, Jian Z L, et al. A high-capacity, low-cost layered sodium manganese oxide material as cathode for sodium-ion batteries. ChemSusChem, 2014, 7(8): 2115 doi: 10.1002/cssc.201402138 [33] Zhao J, Zhao L W, Dimov N, et al. Electrochemical and thermal properties of α-NaFeO2 Cathode for Na-ion batteries. J Electrochem Soc, 2013, 160(5): A3077 doi: 10.1149/2.007305jes [34] Billaud J, Clément R J, Armstrong A R, et al. Β-NaMnO2: A high-performance cathode for sodium-ion batteries. J Am Chem Soc, 2014, 136(49): 17243 doi: 10.1021/ja509704t [35] Piper L F J, Quackenbush N F, Sallis S, et al. Elucidating the nature of pseudo jahn-teller distortions in LixMnPO4: Combining density functional theory with soft and hard X-ray spectroscopy. J Phys Chem C, 2013, 117(20): 10383 doi: 10.1021/jp3122374 [36] Han M H, Gonzalo E, Casas-Cabanas M, et al. Structural evolution and electrochemistry of monoclinic NaNiO2 upon the first cycling process. J Power Sources, 2014, 258: 266 doi: 10.1016/j.jpowsour.2014.02.048 [37] Didier C, Guignard M, Suchomel M R, et al. Thermally and electrochemically driven topotactical transformations in sodium layered oxides NaxVO2. Chem Mater, 2016, 28(5): 1462 doi: 10.1021/acs.chemmater.5b04882 [38] Liu Q N, Hu Z, Chen M Z, et al. P2-type Na2/3Ni1/3Mn2/3O2 as a cathode material with high-rate and long-life for sodium ion storage. J Mater Chem A, 2019, 7(15): 9215 doi: 10.1039/C8TA11927A [39] Yabuuchi N, Kajiyama M, Iwatate J, et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat Mater, 2012, 11(6): 512 doi: 10.1038/nmat3309 [40] Liu Y C, Wang C C, Zhao S, et al. Mitigation of Jahn–Teller distortion and Na+/vacancy ordering in a distorted manganese oxide cathode material by Li substitution. Chem Sci, 2021, 12(3): 1062 doi: 10.1039/D0SC05427E [41] Bai X, Sathiya M, Mendoza-Sánchez B, et al. Anionic redox activity in a newly Zn-doped sodium layered oxide P2-Na2/3Mn1?yZnyO2 (0<y<0.23). Adv Energy Mater, 2018, 8(32): 1802379 doi: 10.1002/aenm.201802379 [42] Gao X, Chen J, Liu H Q, et al. Copper-substituted NaxMO2 (M = Fe, Mn) cathodes for sodium ion batteries: Enhanced cycling stability through suppression of Mn(III) formation. Chem Eng J, 2021, 406: 126830 doi: 10.1016/j.cej.2020.126830 [43] Wang C C, Liu L J, Zhao S, et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat Commun, 2021, 12: 2256 doi: 10.1038/s41467-021-22523-3 [44] Jin T, Wang P F, Wang Q C, et al. Realizing complete solid-solution reaction in high sodium content P2-type cathode for high-performance sodium-ion batteries. Angew Chem Int Ed, 2020, 132(34): 14619 doi: 10.1002/ange.202003972 [45] Zhou C J, Yang L C, Zhou C G, et al. Co-substitution enhances the rate capability and stabilizes the cyclic performance of O3-Type cathode NaNi0.45-xMn0.25Ti0.3CoxO2 for sodium-ion storage at high voltage. ACS Appl Mater Interfaces, 2019, 11(8): 7906 doi: 10.1021/acsami.8b17945 [46] Chen J, Deng W, Gao X, et al. Demystifying the lattice oxygen redox in layered oxide cathode materials of lithium-ion batteries. ACS Nano, 2021, 15(4): 6061 doi: 10.1021/acsnano.1c00304 [47] Jean R. Anion-cation redox competition and the formation of new compounds in highly covalent systems. Chem A Eur J, 1996, 2(9): 1053 doi: 10.1002/chem.19960020904 [48] Assat G, Tarascon J M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat Energy, 2018, 3(5): 373 doi: 10.1038/s41560-018-0097-0 [49] Perez A J, Jacquet Q, Batuk D, et al. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4. Nat Energy, 2017, 2(12): 954 doi: 10.1038/s41560-017-0042-7 [50] Do J, Kim I, Kim H, et al. Towards stable Na-rich layered transition metal oxides for high energy density sodium-ion batteries. Energy Storage Mater, 2020, 25: 62 doi: 10.1016/j.ensm.2019.10.031 [51] Clément R J, Bruce P G, Grey C P. Review—manganese-based P2-type transition metal oxides as sodium-ion battery cathode materials. J Electrochem Soc, 2015, 162(14): A2589 doi: 10.1149/2.0201514jes [52] Mortemard de Boisse B, Liu G D, Ma J T, et al. Intermediate honeycomb ordering to trigger oxygen redox chemistry in layered battery electrode. Nat Commun, 2016, 7: 11397 doi: 10.1038/ncomms11397 [53] Perez A J, Batuk D, Saubanère M, et al. Strong oxygen participation in the redox governing the structural and electrochemical properties of Na-rich layered oxide Na2IrO3. Chem Mater, 2016, 28(22): 8278 doi: 10.1021/acs.chemmater.6b03338 [54] Nanba Y, Iwao T, Boisse B M, et al. Redox potential paradox in NaxMO2 for sodium-ion battery cathodes. Chem Mater, 2016, 28(4): 1058 doi: 10.1021/acs.chemmater.5b04289 [55] Hu Y, Liu T F, Cheng C, et al. Quantification of anionic redox chemistry in a prototype Na-rich layered oxide. ACS Appl Mater Interfaces, 2020, 12(3): 3617 doi: 10.1021/acsami.9b19204 [56] Song S F, Kotobuki M, Zheng F, et al. Y-doped Na2ZrO3: A Na-rich layered oxide as a high-capacity cathode material for sodium-ion batteries. ACS Sustain Chem Eng, 2017, 5(6): 4785 doi: 10.1021/acssuschemeng.7b00196 [57] Du K, Zhu J Y, Hu G R, et al. Exploring reversible oxidation of oxygen in a manganese oxide. Energy Environ Sci, 2016, 9(8): 2575 doi: 10.1039/C6EE01367H [58] Rong X H, Hu E Y, Lu Y X, et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition. Joule, 2019, 3(2): 503 doi: 10.1016/j.joule.2018.10.022 [59] Song B H, Hu E Y, Liu J, et al. A novel P3-type Na2/3Mg1/3Mn2/3O2 as high capacity sodium-ion cathode using reversible oxygen redox. J Mater Chem A, 2019, 7(4): 1491 doi: 10.1039/C8TA09422E [60] Konarov A, Jo J H, Choi J U, et al. Exceptionally highly stable cycling performance and facile oxygen-redox of manganese-based cathode materials for rechargeable sodium batteries. Nano Energy, 2019, 59: 197 doi: 10.1016/j.nanoen.2019.02.042 [61] Shi D R, Wang T, Shadike Z, et al. Anionic redox reaction triggered by trivalent Al3+ in P3-Na0.65Mn0.5Al0.5O2. Chem Commun, 2021, 57(23): 2867 doi: 10.1039/D1CC00373A [62] Zheng W, Liu Q, Wang Z Y, et al. Oxygen redox activity with small voltage hysteresis in Na0.67Cu0.28Mn0.72O2 for sodium-ion batteries. Energy Storage Mater, 2020, 28: 300 doi: 10.1016/j.ensm.2020.03.016 [63] Zhang K, Kim D, Hu Z, et al. Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries. Nat Commun, 2019, 10: 5203 doi: 10.1038/s41467-018-07646-4 [64] Wang Q C, Meng J K, Yue X Y, et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries. J Am Chem Soc, 2019, 141(2): 840 doi: 10.1021/jacs.8b08638 [65] Xiao Y, Zhu Y F, Yao H R, et al. A stable layered oxide cathode material for high-performance sodium-ion battery. Adv Energy Mater, 2019, 9(19): 1803978 doi: 10.1002/aenm.201803978 [66] Xu H, Cheng C, Chu S Y, et al. Anion-cation synergetic contribution to high capacity, structurally stable cathode materials for sodium-ion batteries. Adv Funct Mater, 2020, 30(50): 2005164 doi: 10.1002/adfm.202005164 [67] Zhao C, Yang Q, Geng F, et al. Restraining oxygen loss and boosting reversible oxygen redox in a P2-type oxide cathode by trace anion substitution. ACS Appl Mater Interfaces, 2021, 13(1): 360 doi: 10.1021/acsami.0c16236 [68] Bianchini M, Gonzalo E, Drewett N E, et al. Layered P2–O3 sodium-ion cathodes derived from earth abundant elements. J Mater Chem A, 2018, 6(8): 3552 doi: 10.1039/C7TA11180K [69] Yang L T, Amo J M L, Shadike Z, et al. A Co- and Ni-free P2/O3 biphasic lithium stabilized layered oxide for sodium-ion batteries and its cycling behavior. Adv Funct Mater, 2020, 30(42): 2003364 doi: 10.1002/adfm.202003364 [70] Chen C, Han Z, Chen S, et al. Core-shell layered oxide cathode for high-performance sodium-ion batteries. ACS Appl Mater Interfaces, 2020, 12(6): 7144 doi: 10.1021/acsami.9b19260 [71] Zhao Q Q, Butt F K, Guo Z F, et al. High-voltage P2-type manganese oxide cathode induced by titanium gradient modification for sodium ion batteries. Chem Eng J, 2021, 403: 126308 doi: 10.1016/j.cej.2020.126308 [72] Yang D Z, Liao X Z, Shen J F, et al. A flexible and binder-free reduced graphene oxide/Na2/3[Ni1/3Mn2/3]O2 composite electrode for high-performance sodium ion batteries. J Mater Chem A, 2014, 2(19): 6723 doi: 10.1039/C4TA00682H [73] House R A, Maitra U, Pérez-Osorio M A, et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature, 2020, 577(7791): 502 doi: 10.1038/s41586-019-1854-3 [74] Deng C J, Skinner P, Liu Y Z, et al. Li-substituted layered spinel cathode material for sodium ion batteries. Chem Mater, 2018, 30(22): 8145 doi: 10.1021/acs.chemmater.8b02614 [75] Shen Q Y, Zhao X D, Liu Y C, et al. Dual-strategy of cation-doping and nanoengineering enables fast and stable sodium-ion storage in a novel Fe/Mn-based layered oxide cathode. Adv Sci, 2020, 7(21): 2002199 doi: 10.1002/advs.202002199 [76] Wang Y, Liu Y K, Liu Y C, et al. Recent advances in electrospun electrode materials for sodium-ion batteries. J Energy Chem, 2021, 54: 225 doi: 10.1016/j.jechem.2020.05.065 [77] Wang E H, Niu Y B, Yin Y X, et al. Manipulating electrode/electrolyte interphases of sodium-ion batteries: Strategies and perspectives. ACS Mater Lett, 2021, 3(1): 18 doi: 10.1021/acsmaterialslett.0c00356 [78] Ma L L, Lian F, Zhang F, et al. Research progress of layered Mn-based cathode materials for high-energy-density lithium-ion batteries. Chin J Eng, 2017, 39(2): 167馬磊磊, 連芳, 張帆, 等. 高能量密度鋰離子電池層狀錳基正極材料研究進展. 工程科學學報, 2017, 39(2):167 [79] Zuo W H, Qiu J M, Liu X S, et al. Highly-stable P2-Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering. Energy Storage Mater, 2020, 26: 503 doi: 10.1016/j.ensm.2019.11.024 [80] Kong W J, Wang H B, Sun L M, et al. Understanding the synergic roles of MgO coating on the cycling and rate performance of Na0.67Mn0.5Fe0.5O2 cathode. Appl Surf Sci, 2019, 497: 143814 doi: 10.1016/j.apsusc.2019.143814 [81] Li Y, Shi Q H, Yin X P, et al. Construction nasicon-type NaTi2(PO4)3 nanoshell on the surface of P2-type Na0.67Co0.2Mn0.8O2 cathode for superior room/low-temperature sodium storage. Chem Eng J, 2020, 402: 126181 [82] Moeez I, Susanto D, Ali G, et al. Effect of the interfacial protective layer on the NaFe0.5Ni0.5O2 cathode for rechargeable sodium-ion batteries. J Mater Chem A, 2020, 8(28): 13964 doi: 10.1039/D0TA02837A [83] Wang Y, Tang K, Li X L, et al. Improved cycle and air stability of P3-Na0.65Mn0.75Ni0.25O2 electrode for sodium-ion batteries coated with metal phosphates. Chem Eng J, 2019, 372: 1066 doi: 10.1016/j.cej.2019.05.010 -




 下載:
下載: