Operando X-ray study of service behavior of catalytic materials based on synchrotron radiation
-
摘要: 介紹了基于同步輻射的原位X射線吸收譜、原位X射線衍射譜和原位X射線光電子能譜的基本原理及功能,重點綜述了原位X射線技術在電解水催化材料服役行為動態研究中的應用進展,列舉了多種典型電解水催化劑在反應條件下結構動態變化的研究實例,為實現催化材料全生命周期動態構效關系的精準構建提供了技術基礎。最后,分析總結了原位X射線技術在面臨復雜電化學服役環境時所遇到的問題及挑戰,并提出了對先進同步輻射技術及原位X射線譜學的未來展望。Abstract: Considering the energy and environmental issues faced by human society, hydrogen has become increasingly important, and electrocatalytic water splitting is considered to be an ideal way to solve these energy issues. However, although most electrocatalysts will undergo a structural evolution when in service conditions, our understanding of the service behavior of catalysts is limited. To design highly active catalysts, operando characterization techniques must be used to study their dynamic structural evolution. Today, the development of synchrotron radiation devices has reached an important stage. Synchrotron-radiation-based X-ray characterization, which has high energy, large flux, and excellent collimation compared with the ordinary laboratory X-ray source, can capture the precise structure of catalytic materials. In this review, we present the development status of synchrotron radiation devices and the basic principles of operando X-ray absorption spectroscopy, X-ray diffraction spectroscopy, and X-ray photoelectron spectroscopy based on synchrotron radiation. In addition, we highlight studies related to the dynamic service behavior of water-splitting catalysts under real conditions and list a variety of operando studies of typical water-splitting catalysts, including NiFe hydroxide/(oxy)hydroxides, perovskite oxides, spinel oxides, and noble-metal-based catalysts. The use of operando X-ray techniques deepens our understanding of the catalyst reaction mechanism and provides a basis for identifying the dynamic structure–performance correlation of catalysts. We summarize the problems and challenges of operando X-ray-based techniques in complex electrochemical environments and propose the prospect of an advanced synchrotron radiation facility for operando X-ray characterization. With the development of the next-generation synchrotron radiation facility, adequately using this advanced X-ray light source to study the dynamic structure–activity correlation of catalytic materials throughout their life cycle to achieve the precise design and synthesis of complex pre-catalysts will advance the development of this field by enabling greater refinement and control.
-
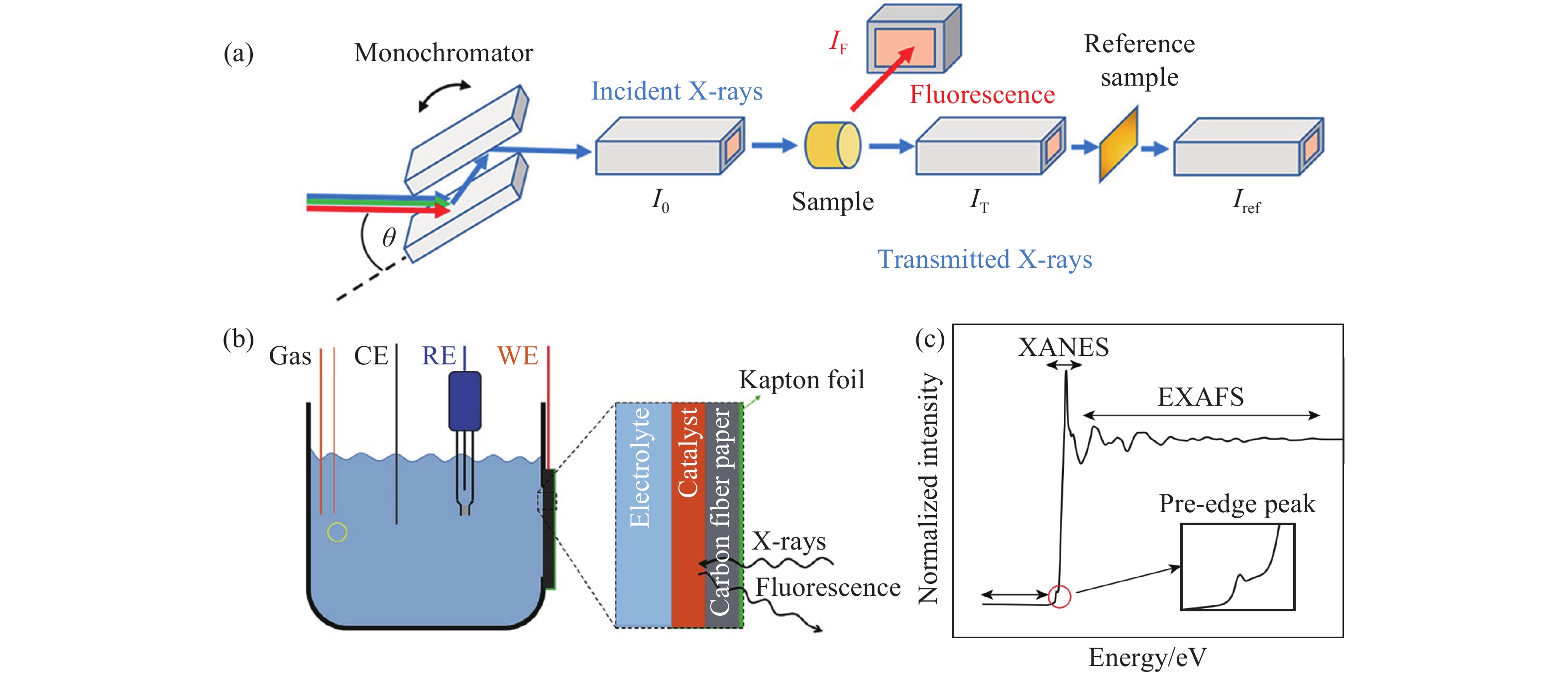
圖 1 (a)XAS裝置示意圖[11];(b)原位XAS電化學反應池裝置示意圖;(c)XAS的圖譜包括吸收前峰,X射線吸收近邊結構和擴展邊X射線吸收精細結構[12]
Figure 1. (a) Schematic of a common setup for X-ray absorption spectroscopy (XAS) measurements[11]; (b) the structure of the electrochemical cell used in operando XAS setup experiments; (c) XAS spectra, including the pre-edge, XANES, and EXAFS regions[12]
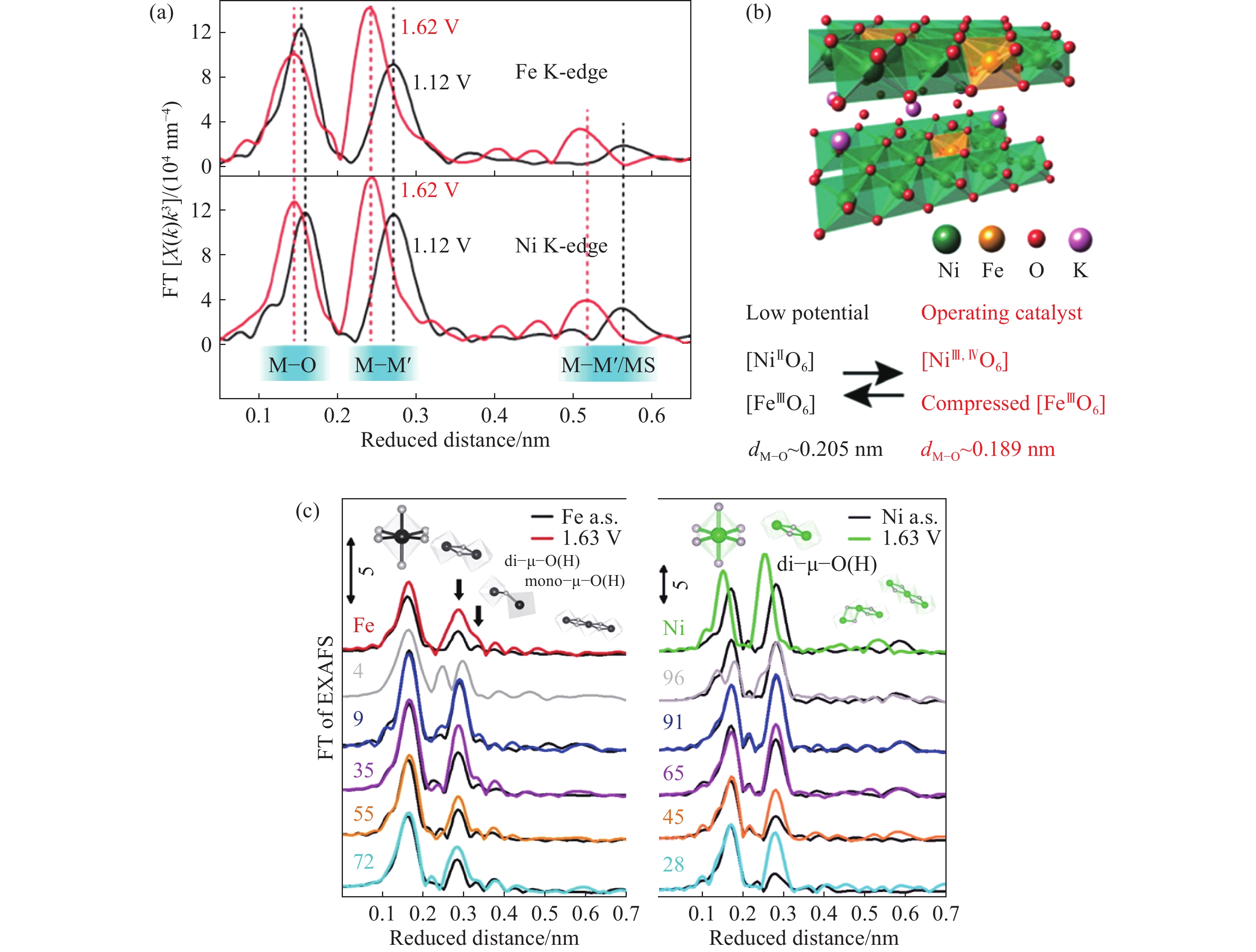
圖 2 (a)Ni和Fe的原位傅里葉轉換EXAFS譜圖;(b)Fe摻雜γ-NiOOH的結構模型[15];(c)不同Fe摻雜量下Ni和Fe位點K邊的傅里葉轉換EXAFS譜圖[18]
Figure 2. (a) Operando Fourier transform–extended X-ray absorption fine structure (FT-EXAFS) results of Fe and Ni sites; (b) structure model of Fe doped γ-NiOOH[15]; (c) Ni K-edge and Fe K-edge FT-EXAFS with different Fe contents[18]
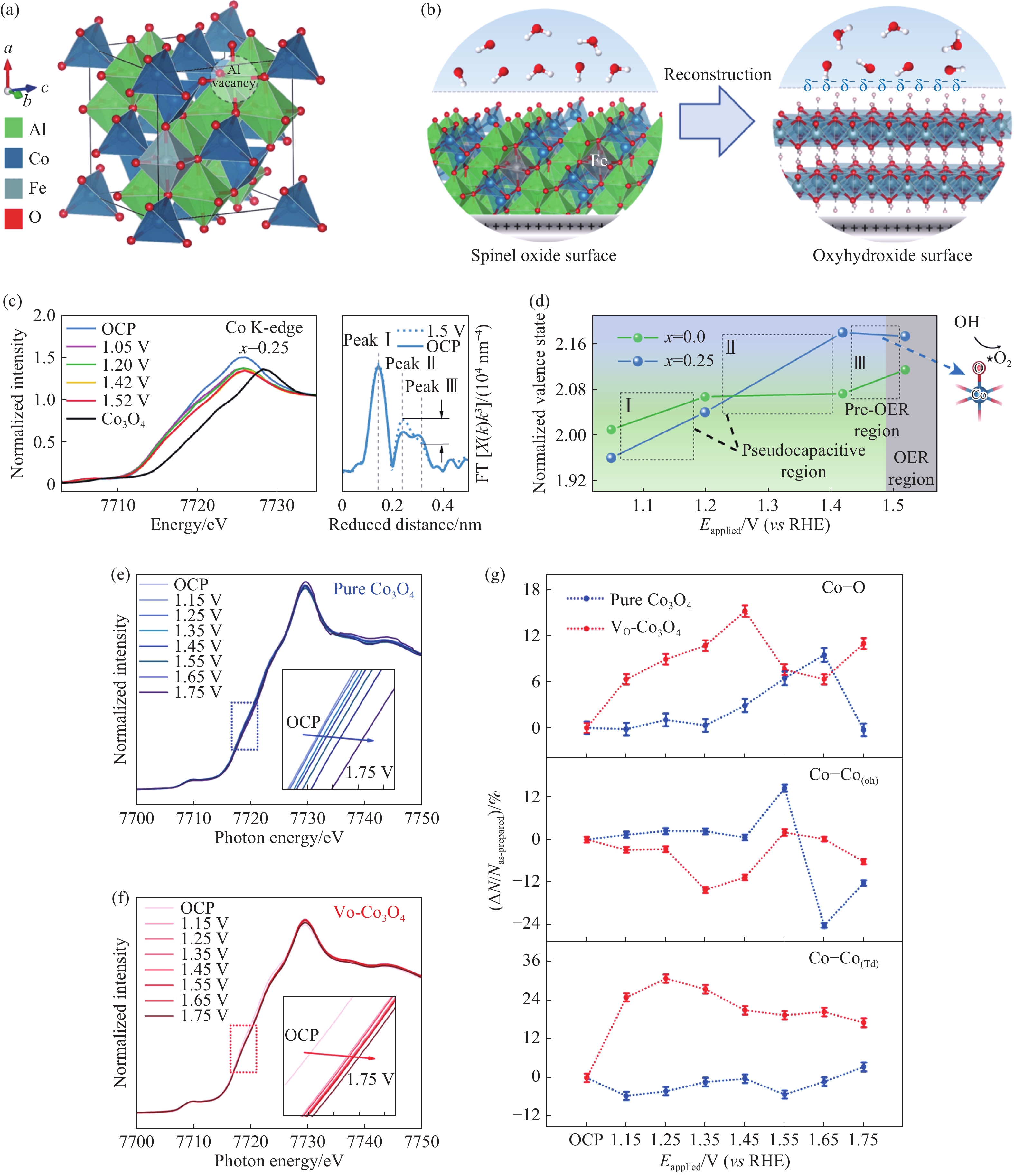
圖 3 (a)帶有Al空位的CoFe0.25Al1.75O4結構模型;(b)CoFe0.25Al1.75O4轉變為CoOOH的過程示意圖;(c)CoFe0.25Al1.75O4中Co位點K邊原位的XANES(左)和傅里葉轉換EXAFS(右)譜圖(OCP為開路電壓);(d)CoFe0.25Al1.75O4和CoAl2O4中Co的氧化態隨施加電壓(Eapplied)的原位變化[21];(e, f)Co3O4和富氧空位Co3O4中Co位點K邊原位的XAFS譜圖;(g)Co位點配位數相對變化ΔN/N隨外加電勢的變化[22]
Figure 3. (a) Model of CoFe0.25Al1.75O4 after Al3+ leaching; (b) reconstruction process from spinel CoFe0.25Al1.75O4 into oxyhydroxide; (c) operando Co K-edge X-ray absorption near-edge structure (XANES) analysis (left axis) and in-situ fourier transforms of Co K-edge extended X-ray absorption fine structure (EXAFS) (right axis) of CoFe0.25Al1.75O4 (OCP is the open circuit potential); (d) operando Co oxidation state of CoFe0.25Al1.75O4 and CoAl2O4 at different potentials applied potentials (Eapplied)[21]; (e, f) operando XAFS of Co K-edge of pure Co3O4 and VO?Co3O4 from OCP to 1.75V; (g) structural coherence change in the EXAFS coordination number of Co ions (ΔN/N) under an applied potential[22]
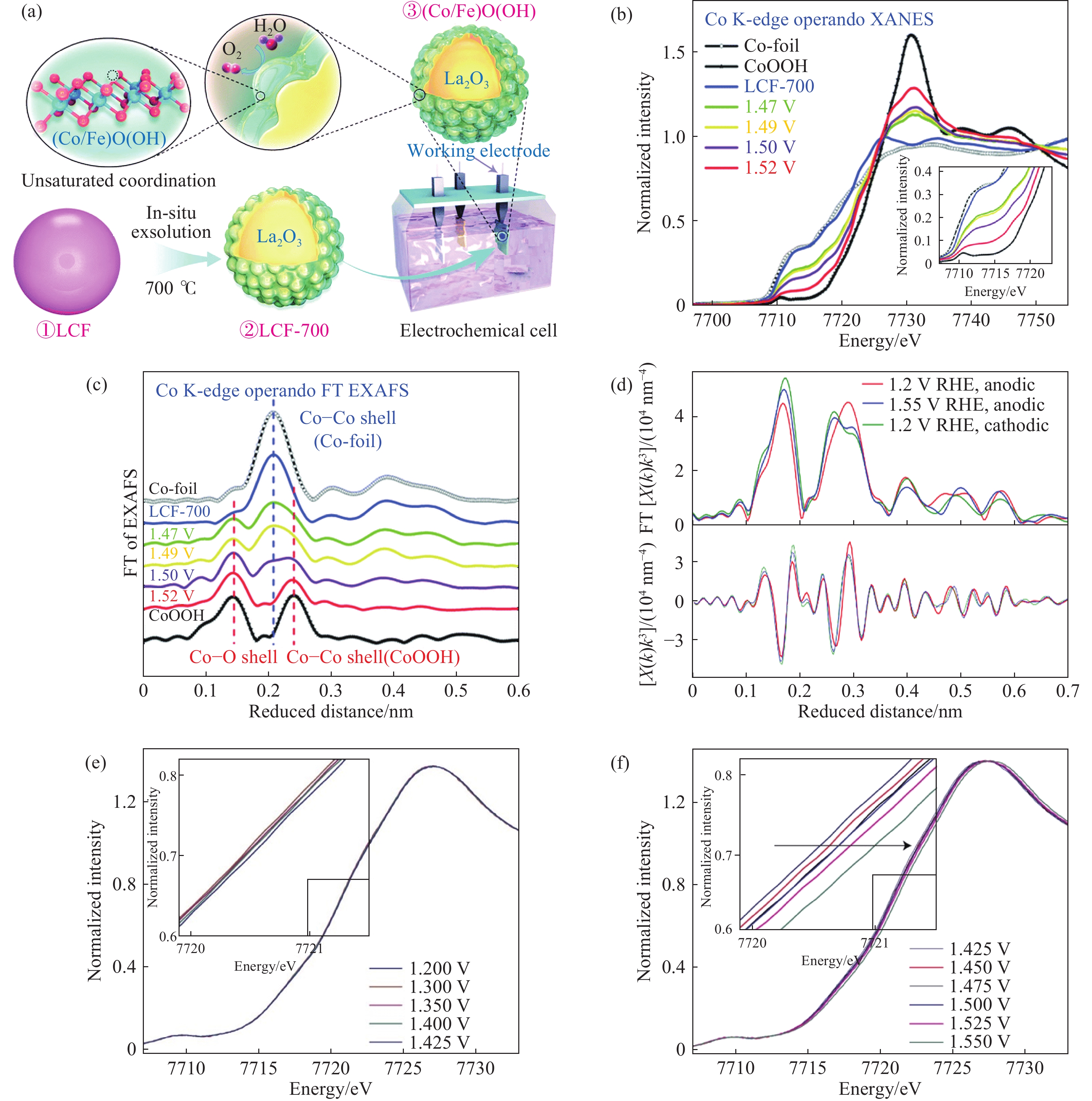
圖 4 (a)形成(Co/Fe)O(OH)過程示意圖;(b, c)LaCo0.8Fe0.2O3-δ中Co位點K邊原位的XANES圖譜和傅里葉轉換EXAFS譜圖[26];(d)Ba0.5Sr0.5Co0.8Fe0.2O3?δ催化劑中Co位點K邊原位的傅里葉轉換EXAFS譜圖;(e, f)催化劑Ba0.5Sr0.5Co0.8Fe0.2O3?δ中Co位點K邊原位的XANES譜圖[28]
Figure 4. (a) Schematic of the formation process of (Co/Fe)O(OH); (b, c) operando Co K-edge X-ray absorption near-edge structure (XANES) and Fourier transform–extended X-ray absorption fine structure (FT-EXAFS) spectra of LaCo0.8Fe0.2O3-δ[26]; (d) operando Co K-edge FT-EXAFS spectra of Ba0.5Sr0.5Co0.8Fe0.2O3?δ electrocatalyst; (e, f) operando XANES spectra recorded at the Co K-edge of the Ba0.5Sr0.5Co0.8Fe0.2O3?δ[28]
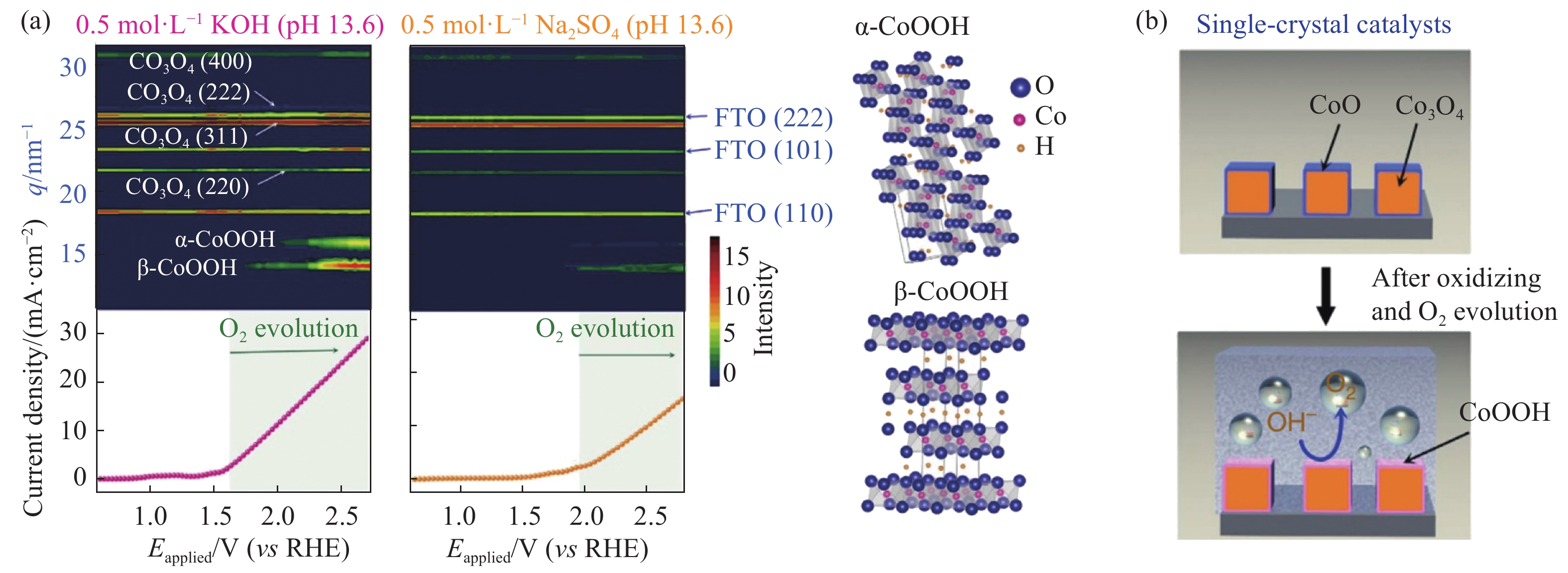
圖 5 (a)單晶Co3O4@CoO催化劑在0.5 mol·L?1 KOH(pH 13.6)和0.5 mol·L?1 Na2SO4(pH 6.5)溶液中的原位掠入射X射線衍射譜圖;(b)單晶Co3O4@CoO催化劑在OER條件下結構轉變的示意圖[29]
Figure 5. (a) Contour plots of in-situ grazing-angle X-ray diffraction signals of a Co3O4@CoO in an aqueous solution containing 0.5 mol·L?1 KOH (pH 13.6) and 0.5 mol·L?1 Na2SO4 (pH 6.5); (b) schematic representation of structural transformation within Co3O4@CoO single-crystal electrocatalysts[29]
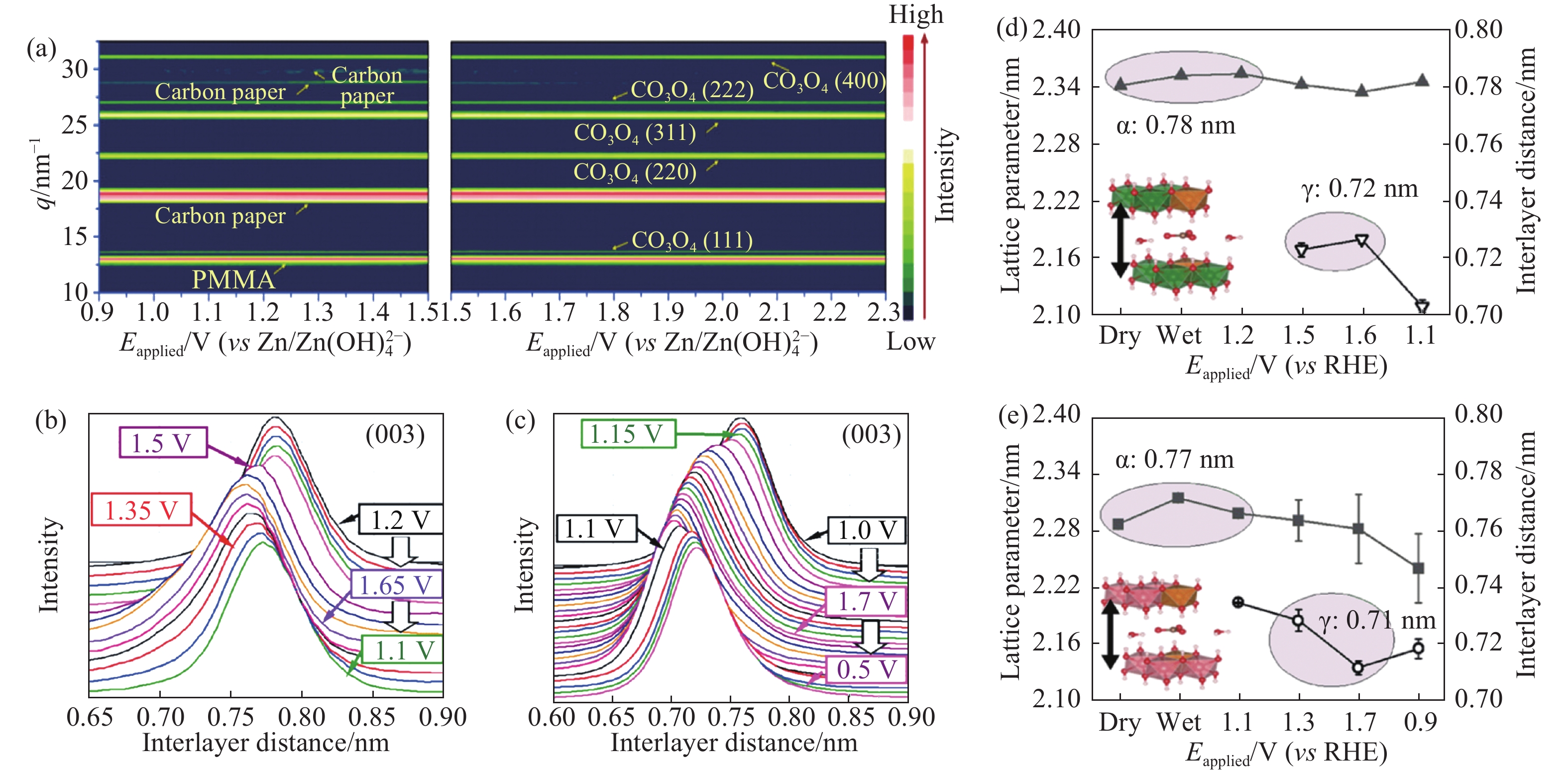
圖 6 (a)Co3O4的原位XRD譜圖[30];(b, c)NiFe和CoFe層狀雙氫氧化物的(003)衍射峰隨著施加電壓的變化;(d, e)NiFe和CoFe層狀雙氫氧化物的層間距隨著施加電壓的變化[31]
Figure 6. (a) Operando X-ray diffraction patterns of Co3O4[30]; (b, c) evolution of (003) peaks of NiFe LDH and CoFe LDH at different potentials; (d, e) evolution of interlayer distances in NiFe LDH and CoFe LDH at different potentials[31]
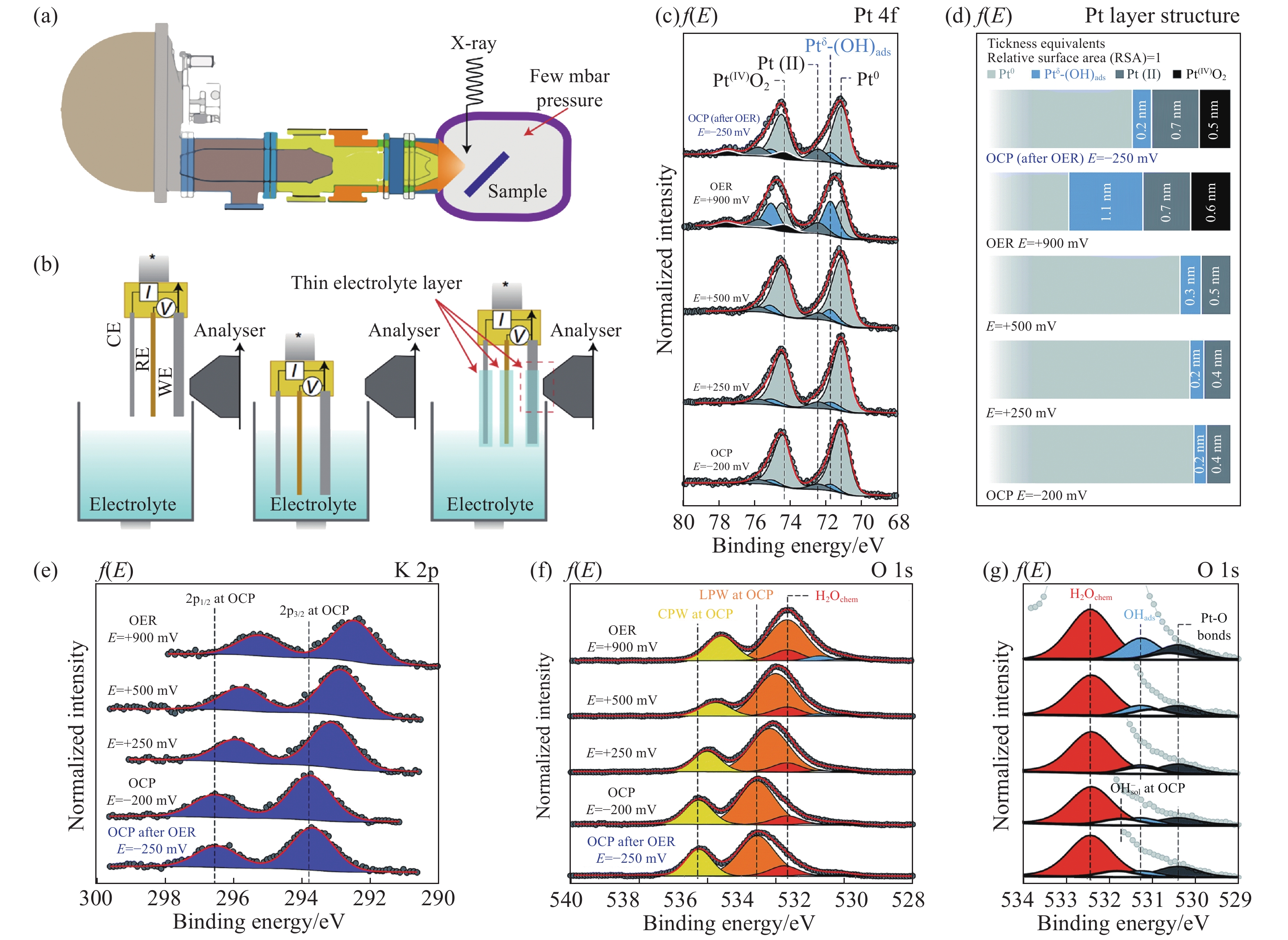
圖 7 (a)近常壓XPS裝置示意圖[40];(b)“浸入?拉出”方法示意圖[43];(c)Pt電極的4f信號隨著施加電壓的變化;(d)Pt電極表面結構隨施加電壓的變化;(e)K 2p信號隨著施加電壓的變化;(f, g)O 1s信號隨著施加電壓的變化及其在低結合能處的放大圖(LPW為液相水;GPW為氣相水)[44]
Figure 7. (a) Schematic of ambient pressure X-ray photoelectron spectroscopy (APXPS) device[40]; (b) illustration of the dip-and-pull operando XPS strategy[43]; (c) Pt 4f spectra as a function of the applied potential; (d) evolution of the Pt surface structure as a function of the applied potential; (e) K 2p photoelectron peak as a function of the applied potential; (f, g) O 1s photoelectron peak as a function of the applied potential and magnification of the low-binding-energy spectrum tail (LPW is the liquid phase water; GPW is the gas phase water)[44]
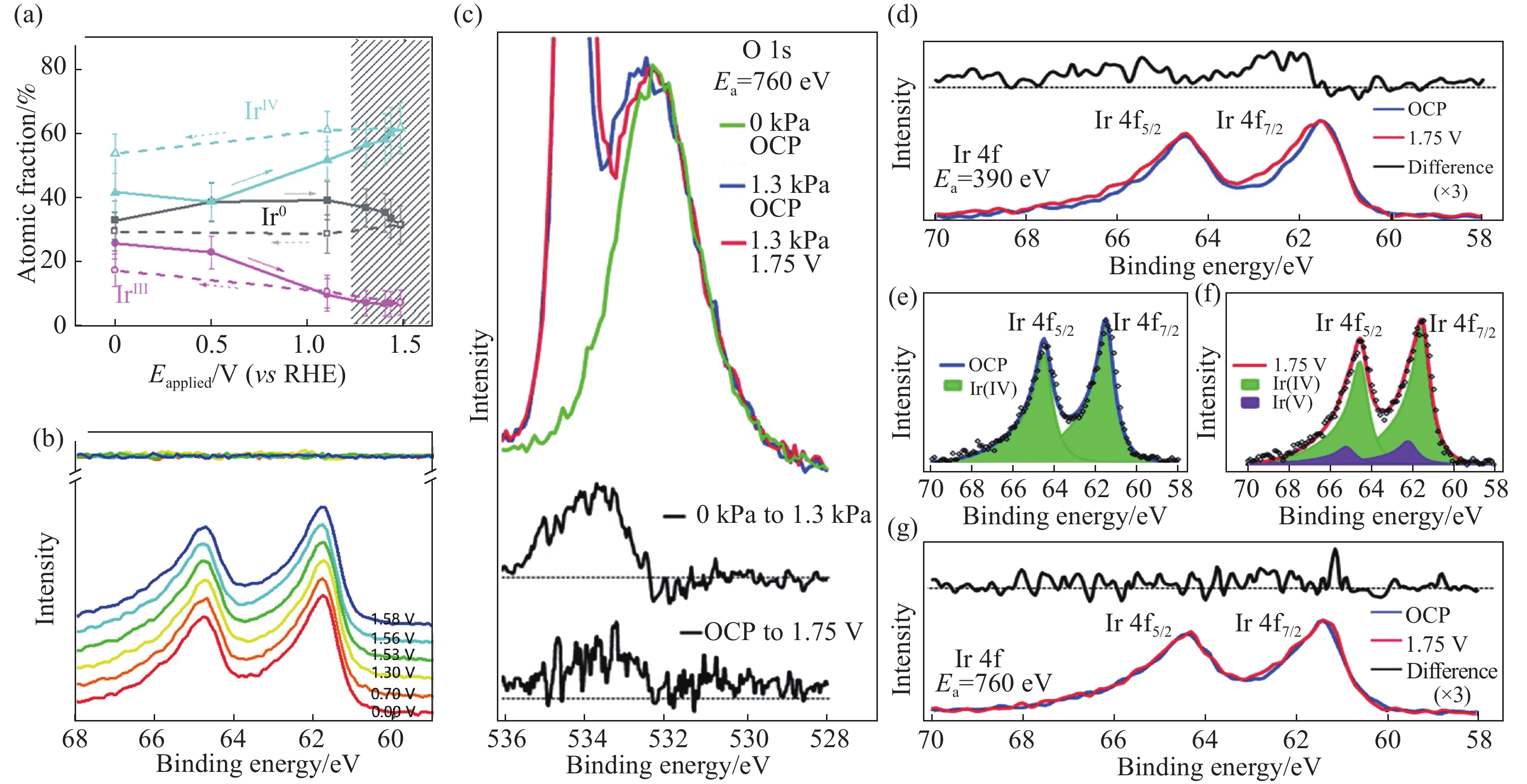
圖 8 (a)Ir0、IrIII和IrIV物種含量隨著電壓的變化;(b)Ir 4f 信號在不同電壓下的位置[46];(c)分別在真空(綠)、1.3 kPa環境壓強(藍)以及OER反應下(紅)的O 1s信號(Ea是實驗時的入射X射線能量);(d~g)IrO2納米顆粒在1.3 kPa水環境壓強下的Ir 4f XPS光譜[47]
Figure 8. (a) Evolutions of Ir0, IrIII and IrIV with the applied potential; (b) Ir 4f X-ray photoelectron spectroscopy (XPS) spectra of the IrO2 anode at different potentials (E?iR): 0 V (red), 0.7 V (orange), 1.3 V (yellow), 1.53 V (green), 1.56 V (cyan), 1.58 V (blue)[46]; (c) O 1s signal measured under vacuum (green) and under 1.3 kPa water pressure at open circuit voltage (blue) and during oxygen evolution reaction (red). The black lines below correspond to the difference spectra (Ea is the incident X-ray energy during the experiment); (d–g) Ir 4f XPS spectra of IrO2 nanoparticles under 1.3 kPa water pressure[47]
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Li X, Zhao L L, Yu J Y, et al. Water splitting: From electrode to green energy system. Nano Micro Lett, 2020, 12(1): 1 doi: 10.1007/s40820-019-0337-2 [2] Hong W T, Risch M, Stoerzinger K A, et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ Sci, 2015, 8(5): 1404 doi: 10.1039/C4EE03869J [3] Zhu Y P, Guo C X, Zheng Y, et al. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc Chem Res, 2017, 50(4): 915 doi: 10.1021/acs.accounts.6b00635 [4] Jiao Y, Zheng Y, Jaroniec M, et al. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem Soc Rev, 2015, 44(8): 2060 doi: 10.1039/C4CS00470A [5] Wang X S, Zheng Y, Sheng W C, et al. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater Today, 2020, 36: 125 doi: 10.1016/j.mattod.2019.12.003 [6] Huang K, Zhu M T, Zhang F P, et al. Preparation of CoP/Co@NPC@rGO nanocomposites with an efficient bifunctional electrocatalyst for hydrogen evolution and oxygen evolution reaction. Chin J Eng, 2020, 42(1): 91黃康, 朱梅婷, 張飛鵬, 等. 一種高效雙功能電催化劑CoP/Co@NPC@rGO的制備. 工程科學學報, 2020, 42(1):91 [7] Xu Z J. Transition metal oxides for water oxidation: All about oxyhydroxides? Sci China Mater, 2020, 63(1): 3 [8] Jiang H L, He Q, Zhang Y K, et al. Structural self-reconstruction of catalysts in electrocatalysis. Accounts Chem Res, 2018, 51(11): 2968 doi: 10.1021/acs.accounts.8b00449 [9] Zhu Y P, Wang J L, Chu H, et al. In situ/operando studies for designing next-generation electrocatalysts. ACS Energy Lett, 2020, 5(4): 1281 doi: 10.1021/acsenergylett.0c00305 [10] Wei C, Feng Z X, Baisariyev M, et al. Valence change ability and geometrical occupation of substitution cations determine the pseudocapacitance of spinel ferrite XFe2O4 (X = Mn, Co, Ni, Fe). Chem Mater, 2016, 28(12): 4129 doi: 10.1021/acs.chemmater.6b00713 [11] Timoshenko J, Roldan Cuenya B. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem Rev, 2021, 121(2): 882 doi: 10.1021/acs.chemrev.0c00396 [12] Wang M Y, árnadóttir L, Xu Z J, et al. In situ X-ray absorption spectroscopy studies of nanoscale electrocatalysts. Nano Micro Lett, 2019, 11(1): 1 doi: 10.1007/s40820-018-0235-z [13] Zhu K Y, Zhu X F, Yang W S. Application of in situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts. Angew Chem Int Ed, 2019, 58(5): 1252 doi: 10.1002/anie.201802923 [14] Lee S, Bai L C, Hu X L. Deciphering iron-dependent activity in oxygen evolution catalyzed by nickel-iron layered double hydroxide. Angew Chem Int Ed, 2020, 59(21): 8072 doi: 10.1002/anie.201915803 [15] Friebel D, Louie M W, Bajdich M, et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J Am Chem Soc, 2015, 137(3): 1305 doi: 10.1021/ja511559d [16] Bates M K, Jia Q Y, Doan H, et al. Charge-transfer effects in Ni? Fe and Ni?Fe?Co mixed-metal oxides for the alkaline oxygen evolution reaction. ACS Catal, 2016, 6(1): 155 doi: 10.1021/acscatal.5b01481 [17] Wang D N, Zhou J G, Hu Y F, et al. In situ X-ray absorption near-edge structure study of advanced NiFe(OH)x electrocatalyst on carbon paper for water oxidation. J Phys Chem C, 2015, 119(34): 19573 doi: 10.1021/acs.jpcc.5b02685 [18] G?rlin M, Chernev P, Ferreira de Araújo J, et al. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni?Fe oxide water splitting electrocatalysts. J Am Chem Soc, 2016, 138(17): 5603 doi: 10.1021/jacs.6b00332 [19] Zheng X L, Zhang B, de Luna P, et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat Chem, 2018, 10(2): 149 doi: 10.1038/nchem.2886 [20] Bergmann A, Martinez-Moreno E, Teschner D, et al. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat Commun, 2015, 6: 8625 doi: 10.1038/ncomms9625 [21] Wu T Z, Sun S N, Song J J, et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat Catal, 2019, 2(9): 763 doi: 10.1038/s41929-019-0325-4 [22] Xiao Z H, Huang Y C, Dong C L, et al. Operando identification of the dynamic behavior of oxygen vacancy-rich Co3O4 for oxygen evolution reaction. J Am Chem Soc, 2020, 142(28): 12087 doi: 10.1021/jacs.0c00257 [23] Suntivich J, May K J, Gasteiger H A, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science, 2011, 334(6061): 1383 doi: 10.1126/science.1212858 [24] Hwang J, Rao R R, Giordano L, et al. Perovskites in catalysis and electrocatalysis. Science, 2017, 358(6364): 751 doi: 10.1126/science.aam7092 [25] May K J, Carlton C E, Stoerzinger K A, et al. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J Phys Chem Lett, 2012, 3(22): 3264 doi: 10.1021/jz301414z [26] Song S Z, Zhou J, Su X Z, et al. Operando X-ray spectroscopic tracking of self-reconstruction for anchored nanoparticles as high-performance electrocatalysts towards oxygen evolution. Energy Environ Sci, 2018, 11(10): 2945 doi: 10.1039/C8EE00773J [27] Fabbri E, Nachtegaal M, Binninger T, et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat Mater, 2017, 16(9): 925 doi: 10.1038/nmat4938 [28] Kim B J, Fabbri E, Abbott D F, et al. Functional role of Fe-doping in Co-based perovskite oxide catalysts for oxygen evolution reaction. J Am Chem Soc, 2019, 141(13): 5231 doi: 10.1021/jacs.8b12101 [29] Tung C W, Hsu Y Y, Shen Y P, et al. Reversible adapting layer produces robust single-crystal electrocatalyst for oxygen evolution. Nat Commun, 2015, 6: 8106 doi: 10.1038/ncomms9106 [30] Wang H Y, Hung S F, Hsu Y Y, et al. In situ spectroscopic identification of μ-OO bridging on spinel Co3O4 water oxidation electrocatalyst. J Phys Chem Lett, 2016, 7(23): 4847 doi: 10.1021/acs.jpclett.6b02147 [31] Dionigi F, Zeng Z H, Sinev I, et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat Commun, 2020, 11(1): 2522 doi: 10.1038/s41467-020-16237-1 [32] Siegbahn H, Siegbahn K. ESCA applied to liquids. J Electron Spectrosc Relat Phenom, 1973, 2(3): 319 doi: 10.1016/0368-2048(73)80023-4 [33] Joyner R W, Roberts M W, Yates K. A “high-pressure” electron spectrometer for surface studies. Surf Sci, 1979, 87(2): 501 doi: 10.1016/0039-6028(79)90544-2 [34] Ruppender H J, Grunze M, Kong C W, et al. In situ X-ray photoelectron spectroscopy of surfaces at pressures up to 1 mbar. Surf Interface Anal, 1990, 15(4): 245 doi: 10.1002/sia.740150403 [35] Ogletree D F, Bluhm H, Hebenstreit E D, et al. Photoelectron spectroscopy under ambient pressure and temperature conditions. Nucl Instrum Methods Phys Res Sect A, 2009, 601(1-2): 151 doi: 10.1016/j.nima.2008.12.155 [36] Kaya S, Ogasawara H, N?slund L ?, et al. Ambient-pressure photoelectron spectroscopy for heterogeneous catalysis and electrochemistry. Catal Today, 2013, 205: 101 doi: 10.1016/j.cattod.2012.08.005 [37] Ogletree D F, Bluhm H, Lebedev G, et al. A differentially pumped electrostatic lens system for photoemission studies in the millibar range. Rev Sci Instrum, 2002, 73(11): 3872 doi: 10.1063/1.1512336 [38] Starr D E, Liu Z, H?vecker M, et al. Investigation of solid/vapor interfaces using ambient pressure X-ray photoelectron spectroscopy. Chem Soc Rev, 2013, 42(13): 5833 doi: 10.1039/c3cs60057b [39] Fadley C S. X-ray photoelectron spectroscopy and diffraction in the hard X-ray regime: Fundamental considerations and future possibilities. Nucl Instrum Methods Phys Res Sect A, 2005, 547(1): 24 doi: 10.1016/j.nima.2005.05.009 [40] Roy K, Artiglia L, van Bokhoven J A. Ambient pressure photoelectron spectroscopy: Opportunities in catalysis from solids to liquids and introducing time resolution. Chem Cat Chem, 2018, 10(4): 666 [41] Stoerzinger K A, Hong W T, Crumlin E J, et al. Insights into electrochemical reactions from ambient pressure photoelectron spectroscopy. Acc Chem Res, 2015, 48(11): 2976 doi: 10.1021/acs.accounts.5b00275 [42] Axnanda S, Crumlin E J, Mao B H, et al. Using “tender” X-ray ambient pressure X-ray photoelectron spectroscopy as A direct probe of solid-liquid interface. Sci Rep, 2015, 5: 9788 doi: 10.1038/srep09788 [43] Handoko A D, Wei F X, Jenndy, et al. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat Catal, 2018, 1(12): 922 doi: 10.1038/s41929-018-0182-6 [44] Favaro M, Valero-Vidal C, Eichhorn J, et al. Elucidating the alkaline oxygen evolution reaction mechanism on platinum. J Mater Chem A, 2017, 5(23): 11634 doi: 10.1039/C7TA00409E [45] Stoerzinger K A, Favaro M, Ross P N, et al. Probing the surface of platinum during the hydrogen evolution reaction in alkaline electrolyte. J Phys Chem B, 2018, 122(2): 864 doi: 10.1021/acs.jpcb.7b06953 [46] Saveleva V, Wang L, Teschner D, et al. Operando evidence for a universal oxygen evolution mechanism on thermal and electrochemical iridium oxides. J Phys Chem Lett, 2018, 9(11): 3154 doi: 10.1021/acs.jpclett.8b00810 [47] Casalongue S H G, Ng M L, Kaya S, et al. In situ observation of surface species on iridium oxide nanoparticles during the oxygen evolution reaction. Angew Chem Int Ed, 2014, 53(28): 7169 doi: 10.1002/anie.201402311 [48] Trotochaud L, Young S L, Ranney J K, et al. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J Am Chem Soc, 2014, 136(18): 6744 doi: 10.1021/ja502379c [49] G?rlin M, de Araújo J F, Schmies H, et al. Tracking catalyst redox states and reaction dynamics in Ni?Fe oxyhydroxide oxygen evolution reaction electrocatalysts: The role of catalyst support and electrolyte pH. J Am Chem Soc, 2017, 139(5): 2070 doi: 10.1021/jacs.6b12250 [50] Ali-L?ytty H, Louie M W, Singh M R, et al. Ambient-pressure XPS study of a Ni?Fe electrocatalyst for the oxygen evolution reaction. J Phys Chem C, 2016, 120(4): 2247 doi: 10.1021/acs.jpcc.5b10931 [51] Favaro M, Drisdell W S, Marcus M A, et al. An operando investigation of (Ni?Fe?Co?Ce)ox system as highly efficient electrocatalyst for oxygen evolution reaction. ACS Catal, 2017, 7(2): 1248 doi: 10.1021/acscatal.6b03126 [52] Favaro M, Yang J H, Nappini S, et al. Understanding the oxygen evolution reaction mechanism on CoOx using operando ambient-pressure X-ray photoelectron spectroscopy. J Am Chem Soc, 2017, 139(26): 8960 doi: 10.1021/jacs.7b03211 -




 下載:
下載: