-
摘要: 黏度是冶金熔渣的基本物理性質,其大小直接影響到反應速率、熔渣分離效果等冶煉過程。通過深入探索熔渣黏度與其結構的關系,在分析熔渣黏度與其(NBO/T)比值(即單個聚合物粒子所擁有的非橋氧數量)相互關系的基礎上,本文提出基于(NBO/T)比值的多元熔渣黏度計算模型。首先建立SiO2–∑MxO簡單渣系的黏度計算模型,通過擬合純氧化物和SiO2–MxO二元渣系的黏度數據得到模型參數,擬合平均誤差在9%~18.5%之間;隨后將該模型擴展至SiO2–Al2O3–∑MxO多元渣系的黏度計算,針對Al2O3在熔渣中同時表現出酸性氧化物和堿性氧化物的特點,在計算SiO2–Al2O3–MxO三元渣系黏度時,將其中的Al2O3拆分為酸性物質和堿性物質來計算(NBO/T)比值和黏度活化能。在SiO2–MxO二元系模型參數的基礎上,通過擬合SiO2–Al2O3–MxO三元渣系的黏度數據得到含Al2O3渣系的模型參數,擬合平均誤差在10%~25%之間。利用該模型計算了SiO2–Al2O3–CaO–MgO–FeO–Na2O–K2O–Li2O–BaO–SrO–MnO多元復雜渣系及其子體系的黏度值,計算平均誤差在25%以內,取得了較好的預報效果。本模型基于熔渣結構理論,并借鑒了經驗模型的數據處理方式,在預報效果和適用范圍上都優于傳統經驗模型,在計算方式上比結構模型要簡單。Abstract: Viscosity is a physical property of fluids and shows resistance to flow. In metallurgical slag, it directly affects various parameters of a metallurgical process such as reaction rate, separation effect, etc. The estimation of viscosity by models during a production process is considered to be much more effective owing to the production fluctuations and complexities of the slag composition. Many viscosity models have been developed in the past, such as the structural model with a wide range of adaptability and complex calculation process and the empirical and semiempirical models with simple structure and a narrow range of adaptability. The present paper proposed a new method to calculate the structural parameter (NBO/T) ratio (the amount of nonbridging oxygen per tetrahedral-coordinated atom), based on which the relationship between the viscosity of molten slag and (NBO/T) ratio was investigated. First, the viscosity model was applied to SiO2–ΣMxO slags, with the model parameters obtained by fitting the viscosity data of pure oxide and SiO2–MxO binary slag, and the average deviations were in the range of 9%–18.5%. Then, the model was extended to calculate the viscosity of SiO2–Al2O3–ΣMxO, a multicomponent complex aluminosilicate system, and Al2O3 was split into acid and basic oxides. Then the oxides were used for calculating the (NBO/T) ratio and viscosity activation energy based on the amphoteric behavior of Al2O3 in SiO2–Al2O3–MxO ternary slag system. Using the parameters of a SiO2–MxO binary system, the model parameters of the Al2O3-containing slag system were obtained by fitting the viscosity data of the SiO2–Al2O3–MxO ternary slag system with average deviations between 10% and 25%. In addition, the viscosity of a multi-complex system (SiO2–Al2O3–CaO–MgO–FeO–Na2O–K2O–Li2O–BaO–SrO–MnO) and its subsystems were also calculated using the model proposed in this paper, and the average deviations is less than 25%, which shows relatively accurate prediction results. The present model is based on the analysis of a slag structure and processing of data by an empirical method. This method has a better prediction effect and wider application range compared with the traditional empirical model, and it uses a simpler calculation process compared with the structure model.
-
Key words:
- degree of the polymerization /
- (NBO/T) ratio /
- viscosity model /
- viscosity /
- slag
-
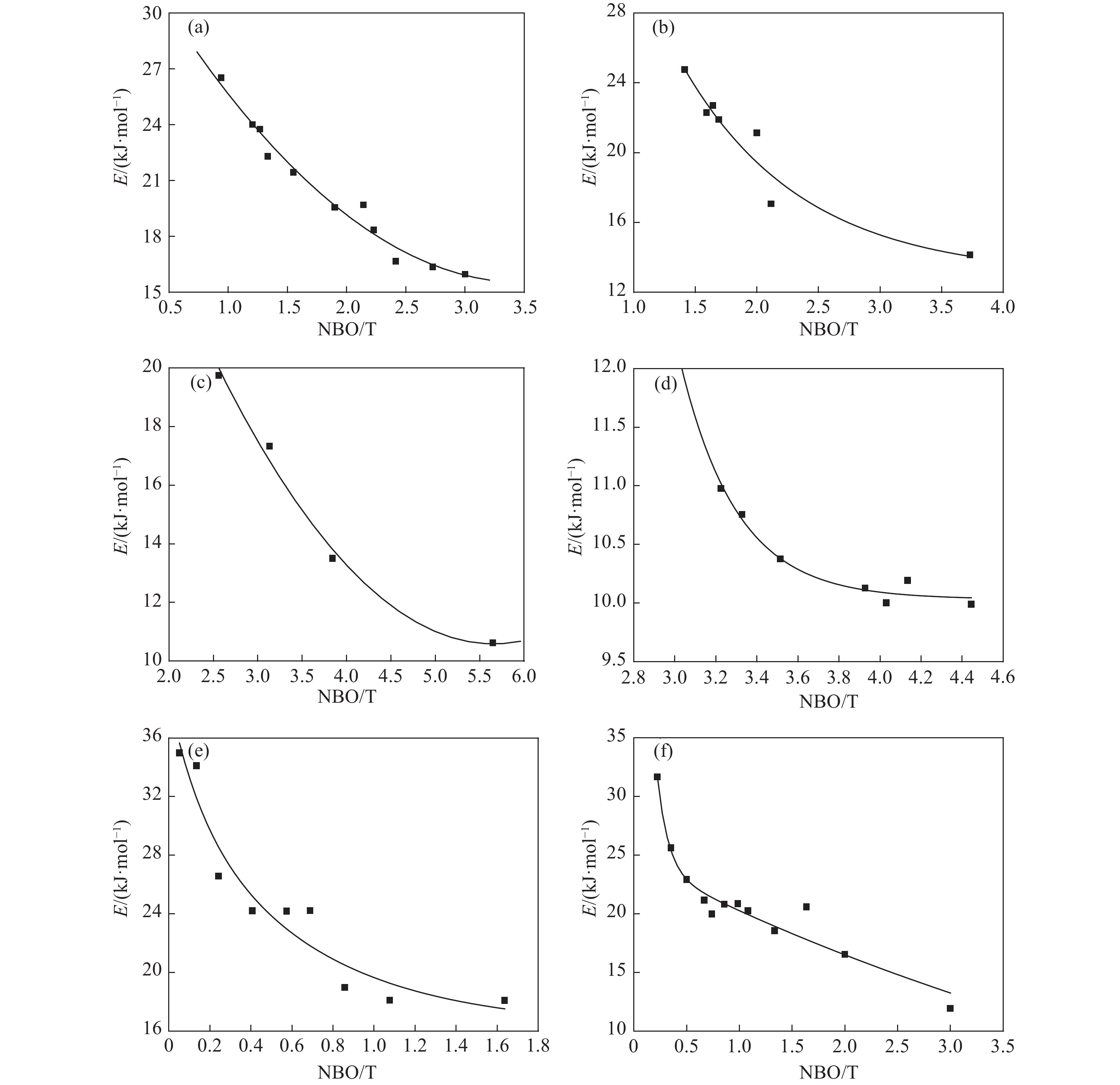
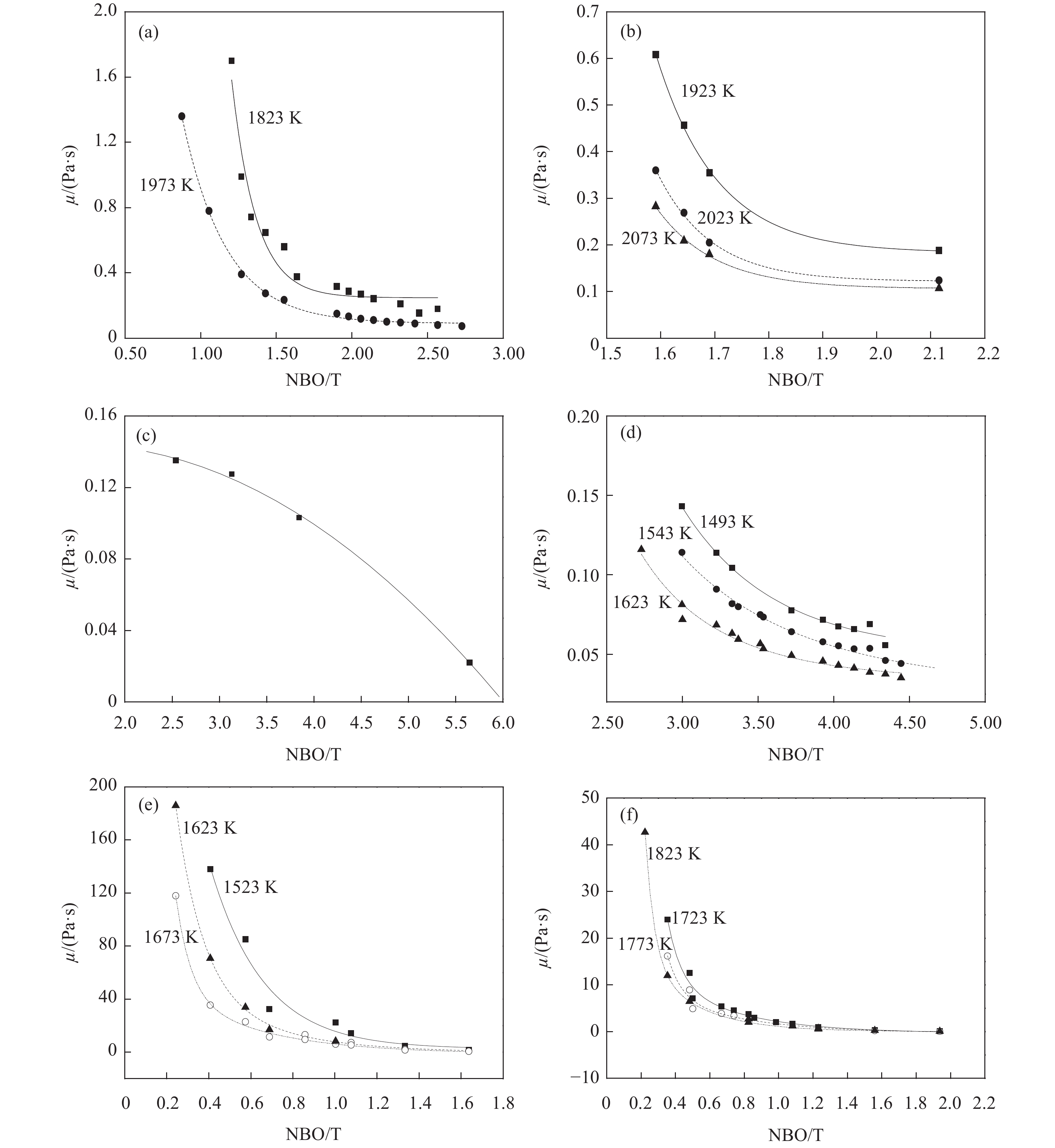
圖 1 SiO2–MxO二元系黏度與(NBO/T)比值的關系。(a) SiO2–CaO[9-11];(b) SiO2–MgO[12];(c) SiO2–MnO[10,13-14];(d) SiO2–FeO[10,15-16];(e) SiO2–K2O[12-17];(f) SiO2–Na2O[11-12]
Figure 1. Relationship between the viscosity and (NBO/T) ratio of SiO2–MxO binary system: (a) SiO2–CaO[9-11];(b) SiO2–MgO[12];(c) SiO2–MnO[10,13-14];(d) SiO2–FeO[10,15-16];(e) SiO2–K2O[12-17];(f) SiO2–Na2O[11-12]
圖 2 SiO2–MxO二元系黏度活化能與(NBO/T)比值的關系。(a) SiO2–CaO[9-11];(b) SiO2–MgO[9-10];(c) SiO2–MnO[10,14];(d) SiO2–FeO[16];(e) SiO2–K2O[12,17];(f) SiO2–Na2O[11,20]
Figure 2. Relationship between the viscosity activation energy and (NBO/T) ratio of SiO2–MxO binary system: (a) SiO2–CaO[9-11];(b) SiO2–MgO[9-10];(c) SiO2–MnO[10,14];(d) SiO2–FeO[16];(e) SiO2–K2O[12,17];(f) SiO2–Na2O[11,20]
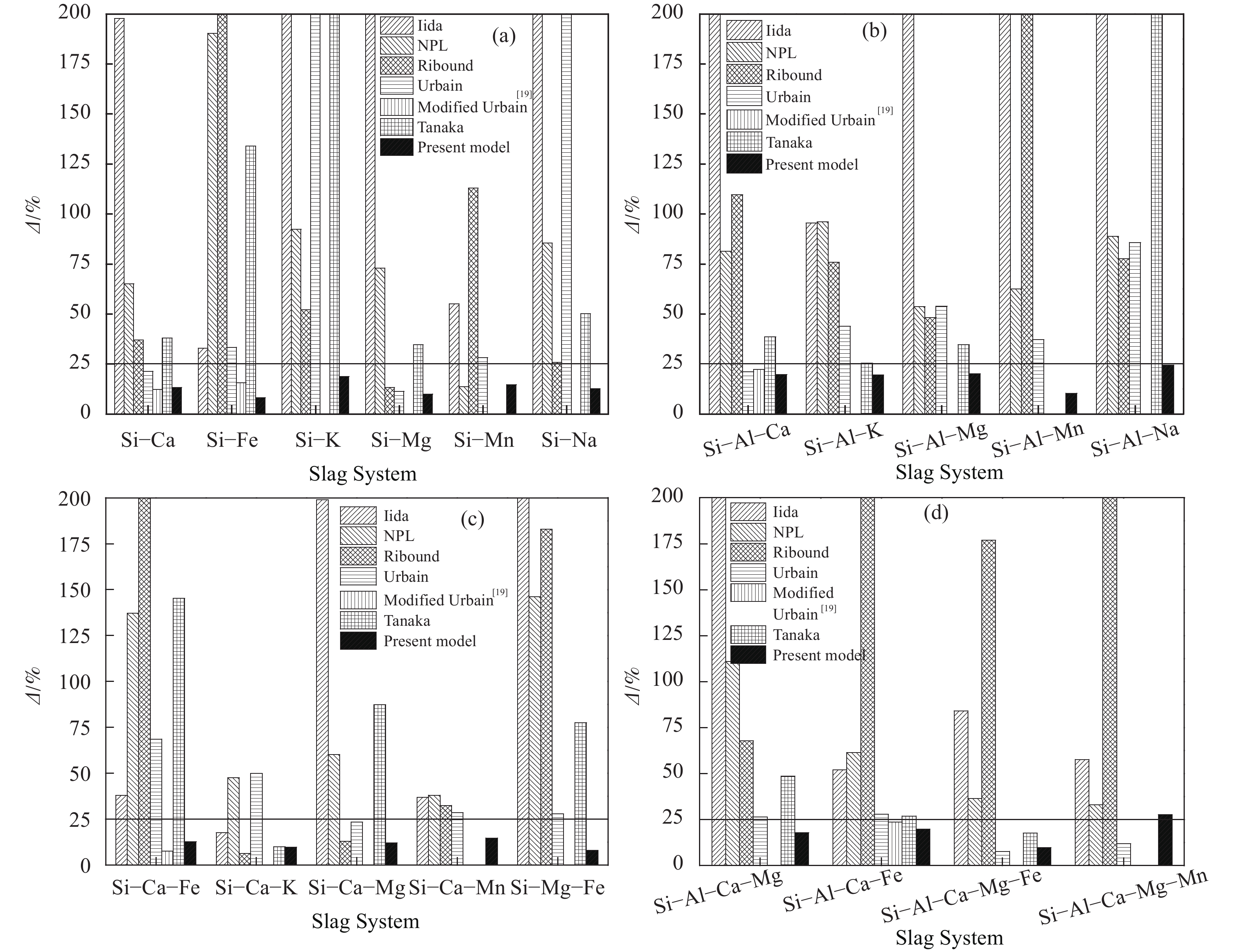
圖 3 黏度模型的預報效果比較。(a) SiO2–MxO二元系;(b) SiO2–Al2O3–MxO三元系;(c) SiO2–CaO/MgO–MxO三元系;(c) SiO2–Al2O3–∑MxO復雜體系
Figure 3. Comparison of prediction effects of the viscosity model: (a) SiO2–MxO binaries slag; (b) SiO2–Al2O3–MxO ternary slag; (c) SiO2–CaO/MgO–MxO ternary slag; (c) SiO2–Al2O3–∑MxO multi–complex slag
表 1 純MxO熔渣的參數
Table 1. Parameters of pure MxO slag
MxO μ/(mPa·s) EMi/(kJ·mol?1) mi 1400 ℃ 1450 ℃ 1500 ℃ 1530 ℃ CaO 23.82 20.67 17.83 16.15 10.703 0.582 MgO 39.66 34.01 28.96 26.03 11.458 0.526 K2O 0.47 0.45 0.43 0.42 4.365 0.543 Na2O 0.83 0.79 0.75 0.72 4.987 0.561 Li2O 3.24 2.96 2.69 2.52 7.53 0.58 MnO 7.35 6.81 6.23 5.79 7.185 0.617 FeO 3.59 3.43 3.07 3.02 6.086 0.626 SrOa) 20.329 0.568 BaOa) 13.303 0.506 a):The m of SrO and BaO are obtained by fitting the viscosities data of corresponding SiO2-MxO binary slag. 表 2 SiO2–MxO二元渣系參數
Table 2. Parameters of SiO2–MxO binary slag
System mass fraction of SiO2/% Temperature range/℃ Ki qi Fitting errors/% SiO2–CaO[9-11,22-24] 40–40 1500–2200 3.18 0.672 11.2 SiO2–MgO[10,12] 45–70 1600–2200 2.633 1.494 9.9 SiO2–FeO[15-16] 25–40 1250–1400 2.303 0.805 8.1 SiO2–MnO[13,22] 20–40 1450–1550 2.333 0.859 14.1 SiO2–K2O[12,17] 40–80 1300–1700 2.556 0.272 18.5 SiO2–Na2O[11-12,20] 40–85 1350–1700 2.901 0.247 11.5 SiO2–Li2O[12,17] 60–80 1250–1700 4.229 0.683 9.3 SiO2–SrO[10,12,17] 35–70 1500–1800 19.156 2.016 10.0 SiO2–BaO[12,17] 25–70 1500–1800 6.403 0.943 16.6 表 3 SiO2–Al2O3–MxO渣系參數
Table 3. Parameters of SiO2–Al2O3–MxO slag
System $R_{{{\rm{M}}_x}{\rm{O1}}} $ $R_{{{\rm{M}}_x}{\rm{O2}}} $ $R_{{{\rm{M}}_x}{\rm{O}}} $ Fitting errors/% SiO2–Al2O3–Li2O[17] 3.92 ?5.721 1.307 11.9 SiO2–Al2O3–Na2O[20,26-27] 2.13 ?0.954 10.904 24.4 SiO2–Al2O3–K2O[17] ?3.666 14.831 ?0.247 19.1 SiO2–Al2O3–SrO[17] 4.519 ?8.03 4.885 11.9 SiO2–Al2O3–MgO[17,29,28-29] 4.792 ?9.176 2.011 19.9 SiO2–Al2O3–CaO[21,23-24,30-32] 3.528 ?4.81 2.268 19.6 SiO2–Al2O3–BaO[17] ?22.607 32.833 ?28.33 16.2 SiO2–Al2O3–MnO[22] 3.233 ?4.811 3.519 10.3 SiO2–Al2O3–FeO*[21,32] 8.671 ?29.49 ?6.376 19.8 *:Utilize the viscosity values of SiO2–Al2O3–CaO–FeO slag system. www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Mao Y W. Metallurgical Melt. Beijing: Metallurgical Industry Press, 1994毛裕文. 冶金熔體. 北京: 冶金工業出版社, 1994 [2] Shankar A, Gornerup M, Lahiri A K, et al. Estimation of viscosity for blast furnace type slags. <italic>Ironmaking Steelmaking</italic>, 2007, 34(6): 477 doi: 10.1179/174328107X17467 [3] Kondratiev A, Hayes P C, Jak E. Development of a quasi–chemical viscosity model for fully liquid slags in the Al<sub>2</sub>O<sub>3</sub>–CaO–'FeO'–MgO–SiO<sub>2</sub> system. The experimental data for the 'FeO'–MgO–SiO<sub>2</sub>, CaO–'FeO'–MgO–SiO<sub>2</sub> and Al<sub>2</sub>O<sub>3</sub>–CaO–'FeO'–MgO–SiO<sub>2</sub> systems at iron saturation. <italic>ISIJ Int</italic>, 2008, 48(1): 7 doi: 10.2355/isijinternational.48.7 [4] Urbain G. Viscosity of silica, alumina, and Na and K oxides: Measurements and estimate. <italic>Revue Internationale des Hautes Temperatures et des Refractaires</italic>, 1985, 22(1): 39 [5] Gaye H, Riboud P V, Welfringer J. Use of a slag model to describe slag–metal reactions and precipitation of inclusions. <italic>Ironmaking Steelmaking</italic>, 1988, 15(6): 319 [6] Forsbacka L, Holappa L, Iida T, et al. Experimental study of viscosities of selected CaO–MgO–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2</sub> slags and application of the Iida model. <italic>Scand J Metall</italic>, 2003, 32(5): 273 doi: 10.1034/j.1600-0692.2003.00652.x [7] Tang X L, Guo M, Wang X D, et al. Estimation model of viscosity based on modified (NBO/T) ratio. <italic>J Univ Sci Technol Beijing</italic>, 2010, 32(12): 1542唐續龍, 郭敏, 王習東, 等. 基于修正的(NBO/T)比值的黏度模型. 北京科技大學學報, 2010, 32(12):1542 [8] Mills K C. Influence of structure on the physico–chemical properties of slags. <italic>ISIJ Int</italic>, 1993, 33(1): 148 doi: 10.2355/isijinternational.33.148 [9] Bockris J O M, Lowe D C. Viscosity and the structure of molten silicates. Proc Royal Soc London. <italic>Ser AMath Phys Sci</italic>, 1954, 226(1167): 423 [10] Urbain G, Bottinga Y, Richet P. Viscosity of liquid silica, silicates and alumino–silicates. <italic>Geochim Cosmochim Acta</italic>, 1982, 46(6): 1061 doi: 10.1016/0016-7037(82)90059-X [11] Mizoguchi K, Yamane M, Suginohara Y. Viscosity measurements of the molten MeO (Me = Ca, Mg, Na)–SiO<sub>2</sub>–Ga<sub>2</sub>O<sub>3</sub> silicate systems. <italic>J Jpn Inst Met</italic>, 1986, 50(1): 76 doi: 10.2320/jinstmet1952.50.1_76 [12] Bockris J O M, Mackenzie J D, Kitchener J A. Viscous flow in silica and binary liquid silicates. <italic>Trans Faraday Soc</italic>, 1955, 51: 1734 doi: 10.1039/tf9555101734 [13] Ji F Z, Du S C, Seetharaman S. Experimental studies of the viscosities in Fe<sub>n</sub>O–MgO–SiO<sub>2</sub> and Fe<sub>n</sub>O–MnO–SiO<sub>2</sub> slags. <italic>Ironmaking Steelmaking</italic>, 1998, 25(4): 309 [14] Segers L, Fontana A, Winand R. Viscosities of melts of silicates of the ternary system CaO–SiO<sub>2</sub>–MnO. <italic>Electrochim Acta</italic>, 1979, 24(2): 213 doi: 10.1016/0013-4686(79)80027-4 [15] Shiraishi Y, Ikeda K, Tamura A, et al. On the viscosity and density of the molten FeO–SiO<sub>2</sub> system. <italic>Trans Jpn Inst Met</italic>, 1978, 19(5): 264 doi: 10.2320/matertrans1960.19.264 [16] Kucharski M, Stubina N M, Toguri J M. Viscosity measurements of molten Fe–O–SiO<sub>2</sub>, Fe–O–CaO–SiO<sub>2</sub>, and Fe–O–MgO–SiO<sub>2</sub> slags. <italic>Can Metall Q</italic>, 1989, 28(1): 7 doi: 10.1179/cmq.1989.28.1.7 [17] Mizoguchi K, Okamoto K, Suginohara Y. Oxygen coordination of Al<sup>3+</sup> ion in several silicate melts studied by viscosity measurements. <italic>J Jpn Inst Met</italic>, 1982, 46(11): 1055 doi: 10.2320/jinstmet1952.46.11_1055溝口數一, 岡本一徳, 杉之原幸夫. 粘度測定による溶融アルミノ珪酸塩中のA1<sup>3+</sup>イオンの酸素配位數について. 日本金屬學會誌, 1982, 46(11):1055 doi: 10.2320/jinstmet1952.46.11_1055 [18] Ray H S, Pal S. Simple method for theoretical estimation of viscosity of oxide melts using optical basicity. <italic>Ironmaking Steelmaking</italic>, 2004, 31(2): 125 doi: 10.1179/030192304225012097 [19] Kondratiev A, Jak E. Review of experimental data and modeling of the viscosities of fully liquid slags in the Al<sub>2</sub>O<sub>3</sub>–CaO–'FeO'–SiO<sub>2</sub> system. <italic>Metall Mater Trans B</italic>:<italic>Process Metall Mater Proc Sci</italic>, 2001, 32(6): 1015 doi: 10.1007/s11663-001-0090-y [20] Kou T, Mizoguchi K, Suginohara Y. The effect of Al<sub>2</sub>O<sub>3</sub> on the viscosity of silicate melts. <italic>J Jpn Inst Met</italic>, 1978, 42(8): 775 doi: 10.2320/jinstmet1952.42.8_775神隆浩, 溝口數一, 杉之原幸夫. 珪酸塩の粘度におよぼすAl<sub>2</sub>O<sub>3</sub>の影響. 日本金屬學會誌, 1978, 42(8):775 doi: 10.2320/jinstmet1952.42.8_775 [21] Allibert M, Gaye H, Geiseler J, et al. Slag Atlas. 2nd Ed. Düsseldorf: Verlag Stahleisen GmbH, 1995 [22] Kawahara M, Mizoguchi K, Suginohara Y. Viscosity and infrared spectra of calcium oxide-silicon dioxide-manganese oxide and manganese oxide-silicon dioxide-aluminum oxide melts. <italic>Bull Kyushu Inst Technol </italic>(<italic>Sci Technol</italic>)<italic></italic>, 1981, 43: 53 [23] Scarfe C M, Cronin D J, Wenzel J T, et al. Viscosity–temperature relationships at 1 atm in the system diopside–anorthite. <italic>Am Mineral</italic>, 1983, 68: 1083 [24] Yasukouchi T, Nakashima K, Mori K. Viscosity of ternary CaO–SiO<sub>2</sub>–M<italic><sub>x</sub></italic>(F, O)<italic><sub>y</sub></italic> and CaO–Al<sub>2</sub>O<sub>3</sub>–Fe<sub>2</sub>O<sub>3</sub> melts. <italic>Tetsu-to-Hagane</italic>, 1999, 85(8): 571 doi: 10.2355/tetsutohagane1955.85.8_571 [25] Mills K C, Chapman L, Fox A B, et al. 'Round robin' project on the estimation of slag viscosities. <italic>Scand J Metall</italic>, 2001, 30(6): 396 doi: 10.1034/j.1600-0692.2001.300608.x [26] Toplis M J, Dingwell D B, Hess K U, et al. Viscosity, fragility, and configurational entropy of melts along the join SiO<sub>2</sub>–NaAlSiO<sub>4</sub>. <italic>Am Mineral</italic>, 1997, 82(9-10): 979 doi: 10.2138/am-1997-9-1014 [27] Toplis M J, Dingwell D B, Lenci T. Peraluminous viscosity maxima in Na<sub>2</sub>O–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2</sub> liquids: the role of triclusters in tectosilicate melts. <italic>Geochim Cosmochim Acta</italic>, 1997, 61(13): 2605 doi: 10.1016/S0016-7037(97)00126-9 [28] Machin J S, Yee T B. Viscosity studies of system CaO–MgO–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2</sub>: IV, 60 and 65% SiO<sub>2</sub>. <italic>J Am Ceram Soc</italic>, 1954, 37(4): 177 doi: 10.1111/j.1151-2916.1954.tb14019.x [29] Machin J S, Yee T B, Hanna D L. Viscosity studies of system CaO–MgO–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2:</sub> Ⅲ, 35, 45, and 50% SiO<sub>2</sub>. <italic>J Am Ceram Soc</italic>, 1952, 35(12): 322 doi: 10.1111/j.1151-2916.1952.tb13057.x [30] Kita Y, Handa A, Iida T. Measurements and calculations of viscosities of blast furnace type slags. <italic>J High Temp Soc</italic>, 2001, 27(4): 144 [31] Machin J S, Yee T B. Viscosity studies of system CaO–MgO–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2</sub>: Ⅱ, CaO–Al<sub>2</sub>O<sub>3</sub>–SiO<sub>2</sub>. <italic>J Am Ceram Soc</italic>, 1948, 31(7): 200 doi: 10.1111/j.1151-2916.1948.tb14290.x [32] Scarfe C M, Cronin D J. Viscosity–temperature relationships of melts at 1 atm in the system diopside–albite. <italic>Am Mineral</italic>, 1986, 71: 767 [33] Tang X L, Ma M S. A description of slag viscosity model and the prediction capability. <italic>China Nonferrous Metall</italic>, 2015, 44(1): 18唐續龍, 馬明生. 熔渣黏度模型介紹及其預報效果比較. 中國有色冶金, 2015, 44(1):18 -




 下載:
下載: