Preparation and properties of Al-rod-Pb-0.2%Ag composite anode by surface ceramization
-
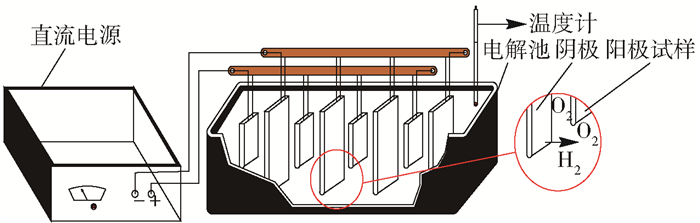
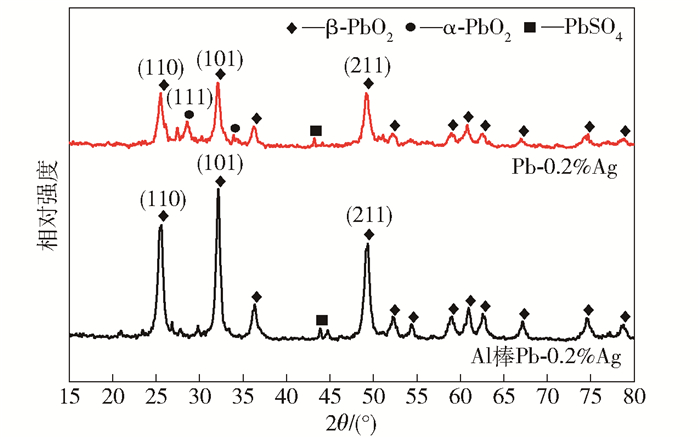
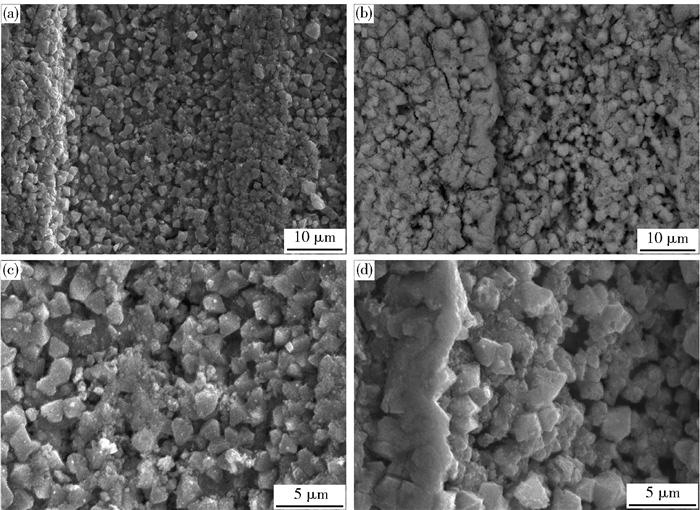
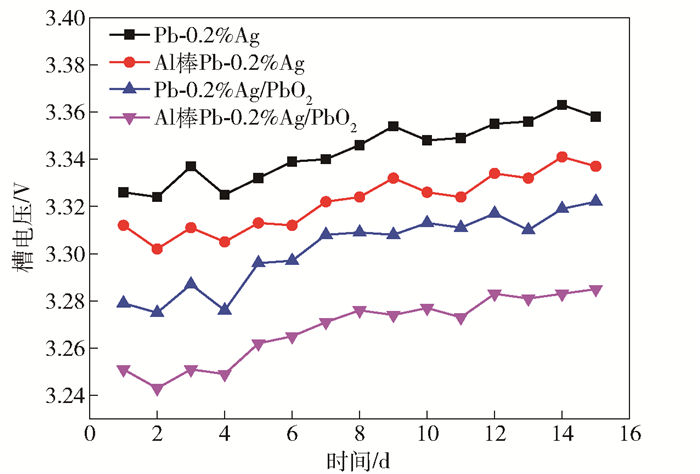
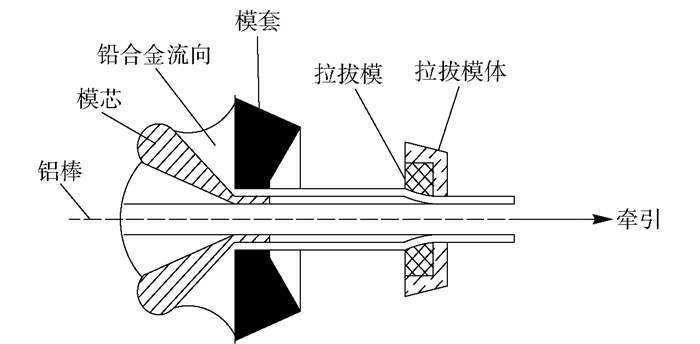
摘要: 為獲得一種鋅電積用低成本、低析氧電位和高催化活性的陽極,在鋁棒表面通過擠壓復合技術包覆Pb-0.2% Ag合金得到Al棒Pb-0.2% Ag陽極.在含氟的硫酸溶液中,通過陽極氧化在Pb-0.2% Ag合金和Al棒Pb-0.2% Ag合金陽極表面形成具有高催化性能的膜層,采用顯微圖像分析儀和數顯顯微硬度計表征了膜層的厚度及硬度,并通過電子拉伸試驗對比了兩種陽極的極限抗拉強度.采用X射線衍射、掃描電子顯微鏡、循環伏安法、陽極極化和交流阻抗法等技術手段研究了Al棒Pb-0.2% Ag與Pb-0.2% Ag陽極表面氧化膜層的物相、形貌以及電化學性能.結果表明:Al棒Pb-0.2% Ag陽極相比Pb-0.2% Ag陽極表面易生成致密較厚的氧化膜層,且膜層硬度提升了41.64%,其氧化膜層主要物相均為電催化活性良好的β-PbO2.新型陽極的極限抗拉強度是傳統陽極的1.3倍,大大改善了陽極材料的機械性能.陽極極化曲線數據顯示Al棒Pb-0.2% Ag/PbO2陽極在電積鋅體系中具有較低的析氧電位(1.35 V vs MSE,500 A·m-2)和較高的交換電流密度(7.079×10-5 A·m-2).循環伏安曲線和交流阻抗數據顯示Al棒Pb-0.2% Ag/PbO2陽極具有較高的電催化活性、較大的表面粗糙度和較小的電荷傳質電阻.在電積鋅實驗中,柵欄型Al棒Pb-0.2% Ag/PbO2陽極相比傳統Pb-0.2% Ag陽極平均槽電壓下降了75 mV,而且大大減少了陽極泥的產生.
-
關鍵詞:
- Al棒Pb-0.2%Ag /
- 表面陶瓷氧化 /
- 鋅電積 /
- 機械性能 /
- 電催化活性
Abstract: To obtain a low-cost anode with low oxygen evolution potential and high catalytic activity for zinc electrowinning, Pb-0.2%Ag alloy was coated on an aluminum matrix surface by extrusion cladding technology, and a film layer with high catalytic performance was formed on the surface of the Pb-0.2%Ag alloy and Al-rod-Pb-0.2%Ag anode by anodization in a fluorine-containing sulfuric acid solution. The thickness and hardness of the film were studied using a microscopic image analyzer and digital microhardness tester, and the ultimate tensile strengths of the two anodes were compared using an electronic tensile tester. The phase, morphology, and electrochemical performance of the Al-rod-Pb-0.2%Ag and Pb-0.2%Ag anode surface film were investigated using X-ray diffractometry, scanning electron microscopy, cyclic voltammetry, anodic polarization, and electrochemical impedance spectroscopy. The results show that the Al-rod-Pb-0.2%Ag anode surface forms a dense and thick oxide film layer more easily than the Pb-0.2%Ag anode and the hardness of the film layer is increased by 41.64%; moreover, the main phase is β-PbO2, and the oxide film layer exhibits good electrocatalytic activity. The ultimate tensile strength of the new anode was 1.3 times that of the traditional anode, which greatly improves the mechanical properties of the anode material. Analytical data of anodic polarization curves reveal that the Al-rod-Pb-0.2%Ag/PbO2 anode shows low oxygen evolution potential (1.35 V vs MSE, 500 A·m-2) and high exchange current density (7.079×10-5 A·m-2) in zinc electrowinning system. Analytical data of cyclic voltammetry and EIS curves indicate that the Al-rod-Pb-0.2%Ag/PbO2 anode has higher electrocatalytic activity, larger surface roughness, and smaller charge transfer resistance. In the zinc electrowinning experiment, the average cell voltage of the fence-like Al-rod-Pb-0.2%Ag/PbO2 anode was 75 mV less than that of the traditional Pb-0.2% Ag anode, and the production of anode slime was greatly reduced. -
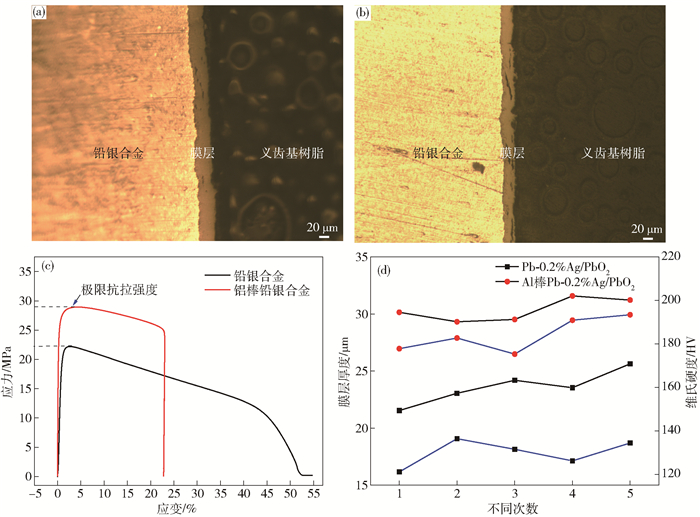
圖 5 不同陽極材料的表面膜層截面金相及力學性能.(a) Al棒Pb-0.2%Ag; (b) Pb-0.2%Ag; (c) 極限抗拉強度; (d) 膜層厚度與維氏硬度
Figure 5. Metallographic and mechanical properties of the surface layer of different anode materials: (a) Al-rod-Pb-0.2%Ag; (b) Pb-0.2%Ag; (c) ultimate tensile strength; (d) Vickers hardness and thickness of surface layer
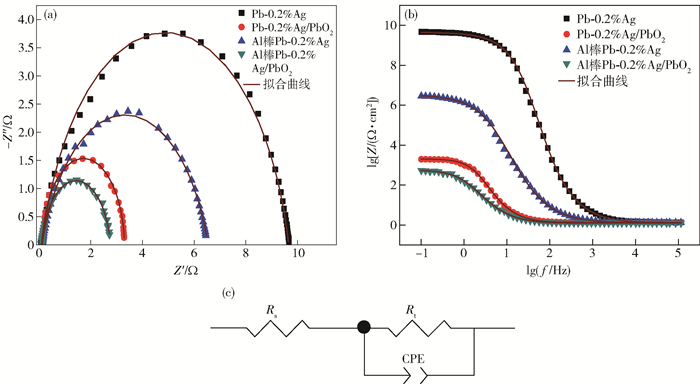
圖 8 不同陽極材料在50 g·L-1 Zn2+, 150 g·L-1 H2SO4溶液中的交流阻抗圖. (a) 阻抗圖譜; (b) Bode圖; (c) 擬合電路圖
Figure 8. EIS spectra of the different anode materials in 50 g·L-1 Zn2+, 150 g·L-1 H2SO4 solution: (a) Nyquist diagrams; (b) Bode plots; (c) electrical equivalent circuit used to simulate impedance data for OER on composite electrode material
表 1 不同陽極材料的析氧反應動力學參數
Table 1. Kinetic parameters of oxygen evolution for the different anode materials
陽極試樣 η/V a1 b1 a2 b2 J0/(A·m-2) 500 A·m-2 1000 A·m-2 Pb-0.2%Ag 0.894 0.951 1.142 0.191 1.266 0.442 1.049×10-6 Pb-0.2%Ag/PbO2 0.794 0.861 1.083 0.222 1.182 0.438 1.323×10-5 Al棒Pb-0.2%Ag 0.874 0.935 1.139 0.204 1.247 0.428 2.610×10-6 Al棒Pb-0.2%Ag/PbO2 0.741 0.819 1.079 0.260 1.171 0.454 7.079×10-5 表 2 不同陽極材料交流阻抗譜的等效電路參數
Table 2. Equivalent circuit parameters of the EIS spectra of the different anode materials
陽極試樣 Rs/(Ω·cm2) Rt/(Ω·cm2) Qdl/(Ω-1·cm-2·sn) n Cdl/(μF·cm-2) RF Pb-0.2%Ag 0.122 9.532 0.001 0.852 198 9.9 Pb-0.2%Ag/PbO2 0.127 3.176 0.020 0.980 17712 885.6 Al棒Pb-0.2%Ag 0.114 6.423 0.007 0.793 1067 53.4 Al棒Pb-0.2%Ag/PbO2 0.088 2.84 0.044 0.898 23308 1165.4 表 3 電積鋅實驗條件
Table 3. Experimental conditions for zinc electrodeposition
www.77susu.com<span id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> <span id="fpn9h"><noframes id="fpn9h"> <th id="fpn9h"></th> <strike id="fpn9h"><noframes id="fpn9h"><strike id="fpn9h"></strike> <th id="fpn9h"><noframes id="fpn9h"> <span id="fpn9h"><video id="fpn9h"></video></span> <ruby id="fpn9h"></ruby> <strike id="fpn9h"><noframes id="fpn9h"><span id="fpn9h"></span> -
參考文獻
[1] Zhang Z, Chen B M, Guo Z C, et al. A review of the novel lead-based anode material used for hydrometallurgy. Mater Rev, 2016, 30(10): 112 https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201619017.htm張璋, 陳步明, 郭忠誠, 等. 濕法冶金中新型鉛基陽極材料的研究進展. 材料導報, 2016, 30(10): 112 https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201619017.htm [2] Mohammadi M, Alfantazi A. The performance of Pb-MnO2 and Pb-Ag anodes in 2 Mn(Ⅱ)-containing sulphuric acid electrolyte solutions. Hydrometallurgy, 2015, 153: 134 doi: 10.1016/j.hydromet.2015.02.009 [3] Clancy M, Bettles C J, Stuart A, et al. The influence of alloying elements on the electrochemistry of lead anodes for electrowinning of metals: a review. Hydrometallurgy, 2013, 131-132: 144 doi: 10.1016/j.hydromet.2012.11.001 [4] Lai Y Q, Jiang L X, Li J, et al. A novel porous Pb-Ag anode for energy-saving in zinc electro-winning: Part I: laboratory preparation and properties. Hydrometallurgy, 2010, 102(1-4): 73 doi: 10.1016/j.hydromet.2010.02.012 [5] Zhong X C, Wang R X, Xu Z F, et al. Influence of Mn2+ on the performance of Pb-Ag anodes in fluoride/chloride-containing H2SO4 solutions. Hydrometallurgy, 2017, 174: 195 doi: 10.1016/j.hydromet.2017.10.014 [6] Paliphot S, Chairuangsri T, Yottawee N, et al. Surface structure of Pb-0.5% Ag anode used in zinc electrowinning. Chiang Mai J Sci, 2006, 33(1): 67 [7] Zhang W S, Cheng C Y. Manganese metallurgy review. Part Ⅲ: Manganese control in zinc and copper electrolytes. Hydrometallurgy, 2007, 89(3-4): 178 doi: 10.1016/j.hydromet.2007.08.011 [8] Rodrigues J, Garbers D, Meyer E H O. Recent developments in the zincor cell house. Can Metall Q, 2001, 40(4): 441 doi: 10.1179/cmq.2001.40.4.441 [9] Ma R X, Cheng S Y, Zhang X Y, et al. Oxygen evolution and corrosion behavior of low-MnO2-content Pb-MnO2 composite anodes for metal electrowinning. Hydrometallurgy, 2016, 159: 6 doi: 10.1016/j.hydromet.2015.10.031 [10] Xu R D, Huang L P, Zhou J F, et al. Effects of tungsten carbide on electrochemical properties and microstructural features of Al/Pb-PANI-WC composite inert anodes used in zinc electrowinning. Hydrometallurgy, 2012, 125-126: 8 doi: 10.1016/j.hydromet.2012.04.012 [11] Zhang Y C, Chen B M, Yang H T, et al. Anodic behavior and microstructure of Al/Pb-Ag anode during zinc electrowinning. Trans Nonferrous Met Soc China, 2014, 24(3): 893 doi: 10.1016/S1003-6326(14)63140-X [12] Yamamoto Y, Fumino K, Ueda M, et al. A potentiodynamic study of the lead electrode in sulphuric acid solution. Electrochim Acta, 1992, 37(2): 199 doi: 10.1016/0013-4686(92)85003-4 [13] Dobrev T, Valchanova I, Stefanov Y, et al. Investigations of new anodic materials for zinc electrowinning. Trans Inst Met Finish, 2009, 87(3): 136 doi: 10.1179/174591909X438938 [14] Yang H T, Guo Z C, Chen B M, et al. Electrochemical behavior of rolled Pb-0.8%Ag anodes in an acidic zinc sulfate electrolyte solution containing Cl- ions. Hydrometallurgy, 2014, 147-148: 148 doi: 10.1016/j.hydromet.2014.05.004 [15] Lai Y Q, Li Y, Jiang L X, et al. Electrochemical behaviors of co-deposited Pb/Pb-MnO2 composite anode in sulfuric acid solution-Tafel and EIS investigations. J Electroanal Chem, 2012, 671: 16 doi: 10.1016/j.jelechem.2012.02.011 [16] Zhang X J, Huang H, Dong J, et al. Influence of manganese on the electrochemical behavior of an aluminum cathode used in zinc electrowinning. Chin J Eng, 2018, 40(7): 800 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201807005.htm張小軍, 黃惠, 董勁, 等. 鋅電積過程中錳元素對鋁陰極的電化學行為影響. 工程科學學報, 2018, 40(7): 800 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201807005.htm [17] Kapalka A, Fóti G, Comninellis C. Determination of the Tafel slope for oxygen evolution on boron-doped diamond electrodes. Electrochem Commun, 2008, 10(4): 607 doi: 10.1016/j.elecom.2008.02.003 [18] Rashkov S, Stefanov Y, Noncheva Z, et al. Investigation of the processes of obtaining plastic treatment and electrochemical behaviour of lead alloys in their capacity as anodes during the electroextraction of zinc Ⅱ. Electrochemical formation of phase layers on binary Pb-Ag and Pb-Ca, and ternary Pb-Ag-Ca alloys in a sulphuric-acid electrolyte for zinc electroextraction. Hydrometallurgy, 1996, 40(3): 319 doi: 10.1016/0304-386X(95)00010-E [19] Lassali T A F, Boodts J F C, Bulhoes L O S. Faradaic impedance investigation of the deactivation mechanism of Ir-based ceramic oxides containing TiO2 and SnO2. J Appl Electrochem, 2000, 30(5): 625 doi: 10.1023/A:1003901520705 [20] Xu L K, Santlebury J D. Microstructure and electrochemical properties of IrO2 Ta2O5-coated titanium anodes. J Electrochem Soc, 2003, 150(6): B254 doi: 10.1149/1.1569479 [21] Tang X, Zhang L, Wang Z, et al. Effect of SO42- on the passive and pitting behavior of 316L austenite stainless steel in a Cl--containing solution. Chin J Eng, 2018, 40(3): 366 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201803013.htm唐嫻, 張雷, 王竹, 等. SO42-對含Cl-溶液中316L奧氏體不銹鋼鈍化行為及點蝕行為的影響. 工程科學學報, 2018, 40(3): 366 https://www.cnki.com.cn/Article/CJFDTOTAL-BJKD201803013.htm [22] Alves V A, da Silva L A, Boodts J F C. Surface characterisation of IrO2/TiO2/CeO2 oxide electrodes and Faradaic impedance investigation of the oxygen evolution reaction from alkaline solution. Electrochim Acta, 1998, 44(8-9): 1525 doi: 10.1016/S0013-4686(98)00276-X -




 下載:
下載: